Abstract
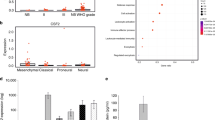
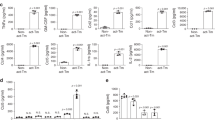
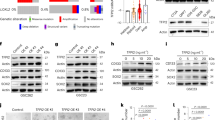
Tumour tissue is infiltrated by myeloid cells that are reprogrammed into alternatively activated/regenerative (M2) macrophages. The contribution of major signalling pathways and their modulators/targets involved in the macrophage reprogramming is poorly known. Glioblastoma (malignant brain tumour) attracts and reprograms brain-resident microglia and peripheral macrophages into cells that increase invasion, angiogenesis and suppress antitumour immunity. Using a ‘function-first’ approach and glioma secretome proteomics we identified osteopontin and lactadherin as proteins that cooperatively activate amoeboid transformation, phagocytosis and motility of primary microglia cultures via integrins and FAK-Akt (focal adhesion kinase-Akt) signalling. A synthetic peptide interfering with integrin ligands blocks glioma–microglia communication, functional activation and M2 gene expression. We found that osteopontin/secreted phosphoprotein 1 (Spp1) produced by non-transformed cells acts as a proinflammatory factor inducing inflammatory signalling and M1 genes, and counteracts the action of lactadherin. Using constructs encoding functional mutants of osteopontin, we demonstrated sequential processing of Spp1 by thrombin and matrix metalloproteinase-3 and/or -7 (MMP-3 and/or -7) in glioma cells, which generates a microglia-activating form devoid of the inflammatory activity, while retaining the M2 reprogramming potential. A similar form of osteopontin is secreted by human glioma cells but not normal human astrocytes. Knockdown of osteopontin or lactadherin in glioma cells reduces intracranial glioma growth, blocks amoeboid transformation of myeloid cells and affects M2 reprogramming of microglia/macrophages. Our findings demonstrate how glioma cells misuse macrophage-activating signals and redesign primarily proinflammatory signals towards their advantage to induce M2 reprogramming of tumour-infiltrating brain macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mantovani A, Sica A . Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010; 22: 231–237.
Noy R, Pollard JW . Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41: 49–61.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20.
Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187: 1157–1165.
Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ . CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009; 284: 34342–34354.
Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol 2010; 185: 642–652.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009; 457: 102–106.
Badie B, Schartner JM . Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery 2000; 46: 957–961; discussion 961–952.
Komohara Y, Ohnishi K, Kuratsu J, Takeya M . Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216: 15–24.
Wei J, Gabrusiewicz K, Heimberger A . The controversial role of microglia in malignant gliomas. Clin Dev Immunol 2013; 2013: 285246.
Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA 2009; 106: 12530–12535.
Sliwa M, Markovic D, Gabrusiewicz K, Synowitz M, Glass R, Zawadzka M et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain 2007; 130: 476–489.
Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B . Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One 2011; 6: e23902.
Hussain SF, Heimberger AB . Immunotherapy for human glioma: innovative approaches and recent results. Expert Rev Anticancer Ther 2005; 5: 777–790.
Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB . The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology 2006; 8: 261–279.
Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB . Innate immune functions of microglia isolated from human glioma patients. J Transl Med 2006; 4: 15.
Sielska M, Przanowski P, Wylot B, Gabrusiewicz K, Maleszewska M, Kijewska M et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol 2013; 230: 310–321.
Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res 2010; 16: 461–473.
Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene 2008; 27: 918–930.
Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-beta1 signaling pathway. J Immunol 2012; 189: 444–453.
Ellert-Miklaszewska A, Dabrowski M, Lipko M, Sliwa M, Maleszewska M, Kaminska B . Molecular definition of the pro-tumorigenic phenotype of glioma-activated microglia. Glia 2013; 61: 1178–1190.
Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol 2003; 54: 388–392.
Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol 2009; 34: 1621–1627.
Held-Feindt J, Hattermann K, Muerkoster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Exp Cell Res 2010; 316: 1553–1566.
Ku MC, Wolf SA, Respondek D, Matyash V, Pohlmann A, Waiczies S et al. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol 2013; 125: 609–620.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF et al. CSF-1 R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 2013; 19: 1264–1272.
Raymond A, Ensslin MA, Shur BD . SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem 2009; 106: 957–966.
Yamaguchi Y, Shao Z, Sharif S, Du XY, Myles T, Merchant M et al. Thrombin-cleaved fragments of osteopontin are overexpressed in malignant glial tumors and provide a molecular niche with survival advantage. J Biol Chem 2013; 288: 3097–3111.
Shao Z, Morser J, Leung LL . Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment. J Biol Chem 2014; 289: 27146–27158.
Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L . Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem 2001; 276: 28261–28267.
Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013; 121: e57–e69.
Anborgh PH, Mutrie JC, Tuck AB, Chambers AF . Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med 2010; 14: 2037–2044.
Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med 2005; 11: 499–506.
Pello OM, De Pizzol M, Mirolo M, Soucek L, Zammataro L, Amabile A et al. Role of c-Myc in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 2011; 119: 411–421.
Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS . Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 2001; 107: 1055–1061.
Smith LL, Cheung HK, Ling LE, Chen J, Sheppard D, Pytela R et al. Osteopontin N-terminal domain contains a cryptic adhesive sequence recognized by alpha9beta1 integrin. J Biol Chem 1996; 271: 28485–28491.
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S . Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417: 182–187.
Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 2009; 114: 49–59.
Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC et al. Osteopontin induces multiple changes in gene expression that reflect the six 'hallmarks of cancer' in a model of breast cancer progression. Mol Carcinogen 2005; 43: 225–236.
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol 2015; 17: 170–182.
Przanowski P, Dabrowski M, Ellert-Miklaszewska A, Kloss M, Mieczkowski J, Kaza B et al. The signal transducers Stat1 and Stat3 and their novel target Jmjd3 drive the expression of inflammatory genes in microglia. J Mol Med (Berl) 2014; 92: 239–254.
Acknowledgements
We thank Beata Kaza and Marcin Sliwa for technical assistance. This work was supported by the Grant 2012/04/A/NZ3/00630 (to BK) from the National Science Center (Poland). PG was supported by GA no. 264173 Bio-Imagine and IP2011013171 from the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Ellert-Miklaszewska, A., Wisniewski, P., Kijewska, M. et al. Tumour-processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene 35, 6366–6377 (2016). https://doi.org/10.1038/onc.2016.55
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.55
This article is cited by
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments
Molecular Cancer (2023)
-
Tumour-derived CSF2/granulocyte macrophage colony stimulating factor controls myeloid cell accumulation and progression of gliomas
British Journal of Cancer (2020)
-
The Retina of Osteopontin deficient Mice in Aging
Molecular Neurobiology (2018)
-
Immune microenvironment of gliomas
Laboratory Investigation (2017)