Abstract
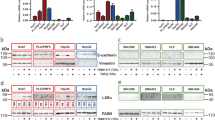
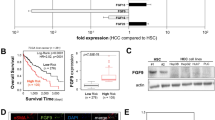
Tumors consistently mimic wound-generating chronic inflammation; however, why they do not heal like wounds with fibrotic scars remains unknown. The components of the tumor microenvironment, such as transforming growth factor β (TGF-β) and fibroblast growth factors (FGFs), may account for this phenomenon. Tumor formation involves continuous activation of the FGF pathway, whereas the repair of tissue injury is a self-limiting process accompanied with controlled activation of the FGF pathway. In the tumor microenvironment TGF-β increases the secretion of FGFs, further promoting the malignant biological properties of tumors. However, during wound healing, sufficient TGF-β together with moderate FGFs lead to matrix deposition and the formation of fibrotic scars. In the present study, TGF-β1 combined with AZD4547, an FGF receptor (FGFR) inhibitor, transformed hepatoma cells into less malignant fibroblast-like cells with respect to morphology, physiological properties, and gene expression profiles. In vivo experiments showed that TGF-β1 combined with AZD4547 not only inhibited tumor growth but also promoted tumor parenchyma fibrosis. Our results indicate that FGFR inhibitor treatment converts the effect of TGF-β on the hepatocellular carcinoma cells from tumor promotion into tumor inhibition by enhancing the induction effect of TGF-β on some fibroblast-associated genes. Converting human liver cancer cells into less malignant fibroblast-like cells and inducing tumor parenchyma cell fibrosis provides an alternative strategy for limiting tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schäfer M, Werner S . Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 2008; 9: 628–638.
Spyrou GE, Naylor IL . The effect of basic fibroblast growth factor on scarring. Br J Plast Surg 2002; 55: 275–282.
Miyazono K, Ehata S, Koinuma D . Tumor-promoting functions of transforming growth factor-β in progression of cancer. Ups J Med Sci 2012; 117: 143–152.
Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudła B . Transforming growth factor β1 (TGFβ1) in physiology and pathology. Endokrynol Pol 2013; 64: 384–396.
Massagué J . TGFβ signalling in context. Nat Rev Mol Cell Biol 2012; 13: 616–630.
Turner N, Grose R . Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2010; 10: 116–129.
Massague J . TGF-beta in Cancer. Cell 2008; 134: 215–230.
Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I et al. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J 2011; 30: 783–795.
Gupta DK, Singh N, Sahu DK . TGF-beta mediated crosstalk between malignant hepatocyte and tumor microenvironment in hepatocellular carcinoma. Cancer Growth Metastasis 2014; 7: 1–8.
Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S et al. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res 2012; 72: 2045–2056.
Chang J, Wang S, Zhang Z, Liu X, Wu Z, Geng R et al. Multiple receptor tyrosine kinase activation attenuates therapeutic efficacy of the fibroblast growth factor receptor 2 inhibitor AZD4547 in FGFR2 amplified gastric cancer. Oncotarget 2015; 6: 2009–2022.
Jia L, Zhang S, Ye Y, Li X, Mercado-Uribe I, Bast RC et al. Paclitaxel inhibits ovarian tumor growth by inducing epithelial cancer cells to benign fibroblast-like cells. Cancer Lett 2012; 326: 176–182.
Kalluri R, Zeisberg M . Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401.
Vasdev N, Nayak NC . CD99 expression in hepatocellular carcinoma: an immunohistochemical study in the fibrolamellar and common variant of the tumour. Indian J Pathol Microbiol 2003; 46: 625–629.
Seol HJ, Chang JH, Yamamoto J, Romagnuolo R, Suh Y, Weeks A et al. Overexpression of CD99 increases the migration and invasiveness of human malignant glioma cells. Genes and Cancer 2012; 3: 535–549.
Krisanaprakornkit S, Chotjumlong P, Pata S, Chruewkamlow N, Reutrakul V, Kasinrerk W . CD99 ligation induces intercellular cell adhesion molecule-1 expression and secretion in human gingival fibroblasts. Arch Oral Biol 2013; 58: 82–93.
Schulte J, Weidig M, Balzer P, Richter P, Franz M, Junker K et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol 2012; 138: 847–860.
Abolhassani A, Riazi GH, Azizi E, Amanpour S, Muhammadnejad S, Haddadi M et al. FGF10: Type III Epithelial Mesenchymal Transition and Invasion in Breast Cancer Cell Lines. J Cancer 2014; 5: 537–547.
Dooley S, Ten Dijke P . TGF-β in progression of liver disease. Cell and Tissue Res 2012; 347: 245–256.
Katsuno Y, Lamouille S, Derynck R . TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 2013; 25: 76–84.
Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, Ozturk M . Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 2010; 52: 966–974.
Arwert EN, Hoste E, Watt FM . Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 2012; 12: 170–180.
El-Assal ON, Yamanoi A, Ono T, Kohno H, Nagasue N . The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin Cancer Res 2001; 7: 1299–1305.
Sandhu D S, Baichoo E, Roberts L R . Fibroblast growth factor signaling in liver carcinogenesis. Hepatology 2014; 59: 1166–1173.
Gaarenstroom T, Hill CS . TGF-β signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin Cell Dev Biol 2014; 32: 107–118.
Jiang W, Zhang Y, Wu H, Zhang X, Gan H, Sun J et al. Role of cross-talk between the Smad2 and MAPK pathways in TGF-beta1-induced collagen IV expression in mesangial cells. Int J Mol Med 2010; 26: 571–576.
Islam SS, Mokhtari RB, El Hout Y, Azadi MA, Alauddin M, Yeger H et al. TGF-β1 induces EMT reprogramming of porcine bladder urothelial cells into collagen producing fibroblasts-like cells in a Smad2/Smad3-dependent manner. J Cell Commun Signal 2013; 8: 39–58.
Macias MJ, Martin-Malpartida P, Massagué J . Structural determinants of Smad function in TGF-β signaling. Trends Biochem Sci 2015; 40: 296–308.
Lavoie H, Therrien M . Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol 2015; 16: 281–298.
Samatar AA, Poulikakos PI . Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov 2014; 13: 928–942.
Thiery JP . Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81473441) and the Program for New Century Excellent Talents in University (NO.NCET-11-1068).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, HR., Wang, XD., Yang, X. et al. An FGFR inhibitor converts the tumor promoting effect of TGF-β by the induction of fibroblast-associated genes of hepatoma cells. Oncogene 36, 3831–3841 (2017). https://doi.org/10.1038/onc.2016.512
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.512
This article is cited by
-
Chemerin reverses the malignant phenotype and induces differentiation of human hepatoma SMMC7721 cells
Archives of Pharmacal Research (2021)
-
Predictive value of serum levels of transforming growth factor beta 1 for the short-term effects of radiotherapy and chemotherapy in patients with esophageal cancer
Oncology and Translational Medicine (2018)