Abstract
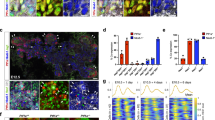
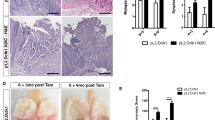
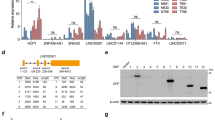
Notch controls pancreatic differentiation during development and is reactivated in pancreatic cancer. In recent years, the importance of Notch signaling in pancreatic tumorigenesis has become increasingly evident; however, it remains unclear how Notch activities are regulated in this context. Here we report differential regulation of Notch receptors by Lunatic Fringe (Lfng), which encodes an O-fucosylpeptide 3-β-N-acetylglucosaminyltransferase known to modify epidermal growth factor repeats in the Notch extracellular domain, during pathogenesis of Kras-induced pancreatic ductal adenocarcinoma (PDAC). We show that Lfng is uniquely expressed in a subset of acinar cells in the adult pancreas. Deletion of Lfng in the KrasLSL-G12D/+;Pdx1-Cre mouse model caused increased activation of Notch3 throughout PDAC initiation and progression, and Notch1 after the onset of disease, associated with marked upregulation of Notch target gene Hes1. Deletion of Lfng also resulted in accumulation of Aldh1-positive cell population. We found that loss of Lfng significantly accelerated Kras-initiated PDAC development and shortened survival of the PDAC mice. Interestingly, Lfng-deficient tumors showed a propensity for a poorly differentiated state with features of epithelial-to-mesenchymal transition. Likewise, knockdown of LFNG in human PDAC cell lines caused elevated Notch activation, associated with either accelerated cell proliferation or expanded Aldh1-positive cell population. Deletion of Lfng resulted in downregulation of Tgfb1, Tgfb2 and Tgfbr2 expression in the wild-type pancreas at all ages examined, and in the KrasLSL-G12D/+;Pdx1-Cre pancreas after PDAC onset, as well as reduced phospho-Smad2 levels in pancreatic tumors. We provide evidence that Lfng regulates transforming growth factor (TGF)-β signaling through Notch-mediated transcriptional repression of TGF-β pathway genes. Taken together, our results reveal a potent tumor-suppressive function for Lfng and crosstalk between Notch and TGF-β pathways in the pancreas, which provides new insight into initiation of PDAC and signals involved in disease progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ryan DP, Hong TS, Bardeesy N . Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049.
Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003; 3: 565–576.
De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci USA 2008; 105: 18907–18912.
Maniati E, Bossard M, Cook N, Candido JB, Emami-Shahri N, Nedospasov SA et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest 2011; 121: 4685–4699.
Mazur PK, Einwachter H, Lee M, Sipos B, Nakhai H, Rad R et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci USA 2010; 107: 13438–13443.
Hanlon L, Avila JL, Demarest RM, Troutman S, Allen M, Ratti F et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res 2010; 70: 4280–4286.
Court H, Amoyel M, Hackman M, Lee KE, Xu R, Miller G et al. Isoprenylcysteine carboxylmethyltransferase deficiency exacerbates KRAS-driven pancreatic neoplasia via Notch suppression. J Clin Invest 2013; 123: 4681–4694.
Ischenko I, Petrenko O, Hayman MJ . Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas. Proc Natl Acad Sci USA 2014; 111: 3466–3471.
Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T et al. Notch signalling controls pancreatic cell differentiation. Nature 1999; 400: 877–881.
Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC . Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol 2012; 362: 57–64.
Murtaugh LC, Stanger BZ, Kwan KM, Melton DA . Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA 2003; 100: 14920–14925.
Shih HP, Kopp JL, Sandhu M, Dubois CL, Seymour PA, Grapin-Botton A et al. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 2012; 139: 2488–2499.
Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 22: 737–750.
Haines N, Irvine KD . Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol 2003; 4: 786–797.
Xu K, Usary J, Kousis PC, Prat A, Wang DY, Adams JR et al. Lunatic Fringe deficiency cooperates with the Met/Caveolin gene amplicon to induce basal-like breast cancer. Cancer Cell 2012; 21: 626–641.
Zhang S, Chung WC, Wu G, Egan SE, Xu K . Tumor-suppressive activity of Lunatic Fringe in prostate through differential modulation of Notch receptor activation. Neoplasia 2014; 16: 158–167.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4: 437–450.
Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 2006; 20: 3130–3146.
Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 2006; 20: 3147–3160.
Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O'Dell MR et al. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res 2012; 72: 4840–4845.
Zhang N, Gridley T . Defects in somite formation in lunatic fringe-deficient mice. Nature 1998; 394: 374–377.
Svensson P, Bergqvist I, Norlin S, Edlund H . MFng is dispensable for mouse pancreas development and function. Mol Cell Biol 2009; 29: 2129–2138.
Mann CD, Bastianpillai C, Neal CP, Masood MM, Jones DJ, Teichert F et al. Notch3 and HEY-1 as prognostic biomarkers in pancreatic adenocarcinoma. PLoS One 2012; 7: e51119.
Doucas H, Mann CD, Sutton CD, Garcea G, Neal CP, Berry DP et al. Expression of nuclear Notch3 in pancreatic adenocarcinomas is associated with adverse clinical features, and correlates with the expression of STAT3 and phosphorylated Akt. J Surg Oncol 2008; 97: 63–68.
Xie MJ, Motoo Y, Su SB, Mouri H, Ohtsubo K, Matsubara F et al. Expression of clusterin in human pancreatic cancer. Pancreas 2002; 25: 234–238.
Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD . Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA 2010; 107: 75–80.
Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B et al. Targeting notch signaling with a notch2/notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res 2015; 21: 2084–2095.
Leung L, Radulovich N, Zhu CQ, Wang D, To C, Ibrahimov E et al. Loss of canonical Smad4 signaling promotes KRAS driven malignant transformation of human pancreatic duct epithelial cells and metastasis. PLoS One 2013; 8: e84366.
Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L et al. TGF-beta: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst 2014. 106 djt369.
Valdez JM, Zhang L, Su Q, Dakhova O, Zhang Y, Shahi P et al. Notch and TGFbeta form a reciprocal positive regulatory loop that suppresses murine prostate basal stem/progenitor cell activity. Cell Stem Cell 2012; 11: 676–688.
Ohnuki H, Jiang K, Wang D, Salvucci O, Kwak H, Sanchez-Martin D et al. Tumor-infiltrating myeloid cells activate Dll4/Notch/TGF-beta signaling to drive malignant progression. Cancer Res 2014; 74: 2038–2049.
Han L, Diehl A, Nguyen N, Korangath P, Teo W, Cho S et al. The Notch pathway inhibits TGF-beta signaling in breast cancer through HEYL-mediated crosstalk. Cancer Res 2014; 74: 6509–6518.
Schiavone M, Rampazzo E, Casari A, Battilana G, Persano L, Moro E et al. Zebrafish reporter lines reveal in vivo signaling pathway activities involved in pancreatic cancer. Dis Model Mech 2014; 7: 883–894.
Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fandrich F . Differential roles of Smad2 and Smad3 in the regulation of TGF-beta1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1. Mol Cancer 2011; 10: 67.
Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM . TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer 2001; 93: 204–211.
Baumgart A, Mazur PK, Anton M, Rudelius M, Schwamborn K, Feuchtinger A et al. Opposing role of Notch1 and Notch2 in a Kras-driven murine non-small cell lung cancer model. Oncogene 2014; 34: 578–588.
Avila JL, Troutman S, Durham A, Kissil JL . Notch1 is not required for acinar-to-ductal metaplasia in a model of Kras-induced pancreatic ductal adenocarcinoma. PLoS One 2012; 7: e52133.
Vo K, Amarasinghe B, Washington K, Gonzalez A, Berlin J, Dang TP . Targeting notch pathway enhances rapamycin antitumor activity in pancreas cancers through PTEN phosphorylation. Mol Cancer 2011; 10: 138.
Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G . Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol 2000; 2: 515–520.
De Waele E, Wauters E, Ling Z, Bouwens L . Conversion of human pancreatic acinar cells toward a ductal-mesenchymal phenotype and the role of transforming growth factor beta and activin signaling. Pancreas 2014; 43: 1083–1092.
Misra K, Luo H, Li S, Matise M, Xiang M . Asymmetric activation of Dll4-Notch signaling by Foxn4 and proneural factors activates BMP/TGFbeta signaling to specify V2b interneurons in the spinal cord. Development 2014; 141: 187–198.
Xu K, Nieuwenhuis E, Cohen BL, Wang W, Canty AJ, Danska JS et al. Lunatic Fringe-mediated Notch signaling is required for lung alveogenesis. Am J Physiol Lung Cell Mol Physiol 2010; 298: L45–L56.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001; 15: 3243–3248.
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4.
Liu H, Kennard S, Lilly B . NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res 2009; 104: 466–475.
Yu X, Alder JK, Chun JH, Friedman AD, Heimfeld S, Cheng L et al. HES1 inhibits cycling of hematopoietic progenitor cells via DNA binding. Stem Cells 2006; 24: 876–888.
Acknowledgements
The authors are thankful to Sean Egan and Tom Gridley for providing mouse strains. We also thank Sean Egan for critical reading of the manuscript. This work was supported in part by NIH grant R21CA175136 and by start-up funds from UMMC Cancer Institute to KX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Zhang, S., Chung, WC. & Xu, K. Lunatic Fringe is a potent tumor suppressor in Kras-initiated pancreatic cancer. Oncogene 35, 2485–2495 (2016). https://doi.org/10.1038/onc.2015.306
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.306
This article is cited by
-
A pan-cancer analysis revealing the role of LFNG, MFNG and RFNG in tumor prognosis and microenvironment
BMC Cancer (2023)
-
Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway
Journal of Experimental & Clinical Cancer Research (2019)