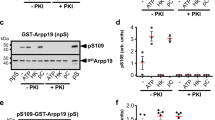
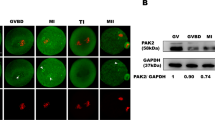
Abstract
Activation of the mitogen-activated protein kinase (MAPK) cascade in mammalian cell lines positively regulates the G2/M transition. The molecular mechanism underlying this biological phenomenon remains poorly understood. Ribosomal S6 kinase (RSK) is a key downstream element of the MAPK cascade. Our previous studies established roles of RSK2 in Cdc25C activation during progesterone-induced meiotic maturation of Xenopus oocytes. In this study we demonstrate that both recombinant RSK and endogenous RSK in Xenopus egg extracts phosphorylate all three isoforms of human Cdc25 at a conserved motif near the catalytic domain. In human HEK293 and PC-3mm2 cell lines, RSK preferentially phosphorylates Cdc25A and Cdc25B in mitotic cells. Phosphorylation of the RSK sites in these Cdc25 isoforms increases their M-phase-inducing activities. Inhibition of RSK-mediated phosphorylation of Cdc25 inhibits G2/M transition. Moreover, RSK is likely to be more active in mitotic cells than in interphase cells, as evidenced by the phosphorylation status of T359/S363 in RSK. Together, these findings indicate that RSK promotes G2/M transition in mammalian cells through activating phosphorylation of Cdc25A and Cdc25B.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Yoon S, Seger R . The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006; 24: 21–44.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007; 1773: 1263–1284.
Roberts PJ, Der CJ . Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26: 3291–3310.
Shaul YD, Seger R . The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 2007; 1773: 1213–1226.
Anjum R, Blenis J . The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 2008; 9: 747–758.
Carriere A, Ray H, Blenis J, Roux PP . The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci 2008; 13: 4258–4275.
Romeo Y, Zhang X, Roux PP . Regulation and function of the RSK family of protein kinases. Biochem J 2012; 441: 553–569.
Alvarez E, Northwood IC, Gonzalez FA, Latour DA, Seth A, Abate C et al. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem 1991; 266: 15277–15285.
Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ . Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem 1992; 267: 24796–24804.
Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ . Myc induces cyclin D1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene 1994; 9: 3635–3645.
Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W et al. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J 1999; 18: 5321–5333.
Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA 2000; 97: 2229–2234.
Brown JR, Nigh E, Lee RJ, Ye H, Thompson MA, Saudou F et al. Fos family members induce cell cycle entry by activating cyclin D1. Mol Cell Biol 1998; 18: 5609–5619.
Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK 3rd et al. pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. J Biol Chem 1999; 274: 7341–7350.
Fox KE, Colton LA, Erickson PF, Friedman JE, Cha HC, Keller P et al. Regulation of cyclin D1 and Wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promoter methylation. J Biol Chem 2008; 283: 35096–35105.
Tamemoto H, Kadowaki T, Tobe K, Ueki K, Izumi T, Chatani Y et al. Biphasic activation of two mitogen-activated protein kinases during the cell cycle in mammalian cells. J Biol Chem 1992; 267: 20293–20297.
Shapiro PS, Vaisberg E, Hunt AJ, Tolwinski NS, Whalen AM, McIntosh JR et al. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol 1998; 142: 1533–1545.
Harding A, Giles N, Burgess A, Hancock JF, Gabrielli BG . Mechanism of mitosis-specific activation of MEK1. J Biol Chem 2003; 278: 16747–16754.
Roberts EC, Shapiro PS, Nahreini TS, Pages G, Pouyssegur J, Ahn NG . Distinct cell cycle timing requirements for extracellular signal-regulated kinase and phosphoinositide 3-kinase signaling pathways in somatic cell mitosis. Mol Cell Biol 2002; 22: 7226–7241.
Liu X, Yan S, Zhou T, Terada Y, Erikson RL . The MAP kinase pathway is required for entry into mitosis and cell survival. Oncogene 2004; 23: 763–776.
Wright JH, Munar E, Jameson DR, Andreassen PR, Margolis RL, Seger R et al. Mitogen-activated protein kinase kinase activity is required for the G(2)/M transition of the cell cycle in mammalian fibroblasts. Proc Natl Acad Sci USA 1999; 96: 11335–11340.
Abbott DW, Holt JT . Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem 1999; 274: 2732–2742.
Knauf JA, Ouyang B, Knudsen ES, Fukasawa K, Babcock G, Fagin JA . Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem 2006; 281: 3800–3809.
Foijer F, Simonis M, van Vliet M, Wessels L, Kerkhoven R, Sorger PK et al. Oncogenic pathways impinging on the G2-restriction point. Oncogene 2008; 27: 1142–1154.
Maller JL . OOcyte maturation in amphibians. Dev Biol (N Y 1985) 1985; 1: 289–311.
Castro A, Peter M, Lorca T, Mandart E . c-Mos and cyclin B/cdc2 connections during Xenopus oocyte maturation. Biol Cell 2001; 93: 15–25.
Palmer A, Nebreda AR . The activation of MAP kinase and p34cdc2/cyclin B during the meiotic maturation of Xenopus oocytes. Prog Cell Cycle Res 2000; 4: 131–143.
Gebauer F, Richter JD . Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis. Bioessays 1997; 19: 23–28.
Gotoh Y, Nishida E . Activation mechanism and function of the MAP kinase cascade. Mol Reprod Dev 1995; 42: 486–492.
Palmer A, Gavin AC, Nebreda AR . A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. Embo J 1998; 17: 5037–5047.
Mueller PR, Coleman TR, Kumagai A, Dunphy WG . Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 1995; 270: 86–90.
Wang R, He G, Nelman-Gonzalez M, Ashorn CL, Gallick GE, Stukenberg PT et al. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell 2007; 128: 1119–1132.
Kumagai A, Dunphy WG . The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell 1991; 64: 903–914.
Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW . cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 1991; 67: 197–211.
Gabrielli BG, Lee MS, Walker DH, Piwnica-Worms H, Maller JL . Cdc25 regulates the phosphorylation and activity of the Xenopus cdk2 protein kinase complex. J Biol Chem 1992; 267: 18040–18046.
Wang R, Jung SY, Wu CF, Qin J, Kobayashi R, Gallick GE et al. Direct roles of the signaling kinase RSK2 in Cdc25C activation during Xenopus oocyte maturation. Proc Natl Acad Sci USA 2010; 107: 19885–19890.
Bhatt RR, Ferrell JE Jr . Cloning and characterization of Xenopus Rsk2, the predominant p90 Rsk isozyme in oocytes and eggs. J Biol Chem 2000; 275: 32983–32990.
Nurse P . Universal control mechanism regulating onset of M-phase. Nature 1990; 344: 503–508.
Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH . p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 2005; 169: 227–231.
Davis FM, Tsao TY, Fowler SK, Rao PN . Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA 1983; 80: 2926–2930.
Lee MS, Ogg S, Xu M, Parker LL, Donoghue DJ, Maller JL et al. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell 1992; 3: 73–84.
Rime H, Huchon D, De Smedt V, Thibier C, Galaktionov K, Jessus C et al. Microinjection of Cdc25 protein phosphatase into Xenopus prophase oocyte activates MPF and arrests meiosis at metaphase I. Biol Cell 1994; 82: 11–22.
Karlsson C, Katich S, Hagting A, Hoffmann I, Pines J . Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J Cell Biol 1999; 146: 573–584.
Cazales M, Schmitt E, Montembault E, Dozier C, Prigent C, Ducommun B . CDC25B phosphorylation by Aurora-A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle 2005; 4: 1233–1238.
Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci 117 (Pt 12): 2523–2531.
Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal 2010; 3: ra64.
Pearce LR, Komander D, Alessi DR . The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010; 11: 9–22.
Hauge C, Frodin M . RSK and MSK in MAP kinase signalling. J Cell Sci 2006; 119 (Pt 15): 3021–3023.
Boutros R, Dozier C, Ducommun B . The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 2006; 18: 185–191.
Boutros R, Lobjois V, Ducommun B . CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 2007; 7: 495–507.
Aressy B, Ducommun B . Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med Chem 2008; 8: 818–824.
Chen Y, Poon RY . The multiple checkpoint functions of CHK1 and CHK2 in maintenance of genome stability. Front Biosci 2008; 13: 5016–5029.
Reinhardt HC, Yaffe MB . Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 2009; 21: 245–255.
Donzelli M, Draetta GF . Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep 2003; 4: 671–677.
Aressy B, Bugler B, Valette A, Biard D, Ducommun B . Moderate variations in CDC25B protein levels modulate the response to DNA damaging agents. Cell Cycle 2008; 7: 2234–2240.
Cangi MG, Piccinin S, Pecciarini L, Talarico A, Dal Cin E, Grassi S et al. Constitutive overexpression of CDC25A in primary human mammary epithelial cells results in both defective DNA damage response and chromosomal breaks at fragile sites. Int J Cancer 2008; 123: 1466–1471.
Varmeh S, Manfredi JJ . Overexpression of the dual specificity phosphatase, Cdc25C, confers sensitivity on tumor cells to doxorubicin-induced cell death. Mol Cancer Ther 2008; 7: 3789–3799.
Clark DE, Errington TM, Smith JA, Frierson HF Jr., Weber MJ, Lannigan DA . The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res 2005; 65: 3108–3116.
Kang S, Elf S, Lythgoe K, Hitosugi T, Taunton J, Zhou W et al. p90 ribosomal S6 kinase 2 promotes invasion and metastasis of human head and neck squamous cell carcinoma cells. J Clin Invest 2010; 120: 1165–1177.
Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM . Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res 2003; 63: 8330–8337.
Denko NC, Giaccia AJ, Stringer JR, Stambrook PJ . The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc Natl Acad Sci USA 1994; 91: 5124–5128.
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA . Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 2008; 11: 32–50.
Nam HJ, Kim S, Lee MW, Lee BS, Hara T, Saya H et al. The ERK-RSK1 activation by growth factors at G2 phase delays cell cycle progression and reduces mitotic aberrations. Cell Signal 2008; 20: 1349–1358.
Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P . Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem 1998; 273: 1496–1505.
Acknowledgements
This work was supported by the Department of Defense (DOD) Prostate Cancer Research Program (PCRP) grants W81XWH-09-1-0274 (JK) and W81XWH-09-1-0272 (S-HL), by grant 30300173 from the National Natural Science Foundation of China and grant 20071D0503100293 from the Beijing Talents Foundation (WZ), and a PCF Challenge grant and NCI CA140388 (GEG). DNA sequencing was performed by the DNA Analysis Facility of UT MD Anderson Cancer Center supported by NCI grant CA 16672. The Translational Chemistry Core Facility at the MD Anderson Cancer Center was supported by the Cancer Center support grant CA016672.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, C., Liu, S., Lee, YC. et al. RSK promotes G2/M transition through activating phosphorylation of Cdc25A and Cdc25B. Oncogene 33, 2385–2394 (2014). https://doi.org/10.1038/onc.2013.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.182
Keywords
This article is cited by
-
The Role of p90 Ribosomal S6 Kinase (RSK) in Tyrosine Kinase Inhibitor (TKI)-Induced Cardiotoxicity
Journal of Cardiovascular Translational Research (2023)
-
Combination of RSK inhibitor LJH-685 and FLT3 inhibitor FF-10101 promoted apoptosis and proliferation inhibition of AML cell lines
Cellular Oncology (2022)
-
The role of the p90 ribosomal S6 kinase family in prostate cancer progression and therapy resistance
Oncogene (2021)
-
MicroRNA-211, a direct negative regulator of CDC25B expression, inhibits triple-negative breast cancer cells’ growth and migration
Tumor Biology (2015)
-
High expression of Cdc25B and low expression of 14-3-3σ is associated with the development and poor prognosis in urothelial carcinoma of bladder
Tumor Biology (2014)