Abstract
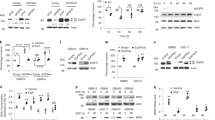
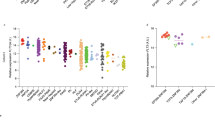
Amplification and rearrangements of the epidermal growth factor receptor (EGFR) gene are frequently found in glioblastoma multiforme (GBM). The most common variant is EGFR variant III (EGFRvIII). Research suggests that EGFRvIII could be a marker for a cancer stem cell or tumor-initiating population. If amplification and rearrangement are early events in tumorigenesis, this implies that they should be preserved throughout the tumor. However, in primary GBM, EGFRvIII expression is focal and sporadic. Unexpectedly, we found EGFR amplification and rearrangement throughout the tumor, including regions with no EGFRvIII expression, suggesting that mechanisms exist to modulate EGFRvIII expression even in the presence of high gene amplification. To study this phenomenon, we characterized three GBM cell lines with endogenous EGFRvIII. EGFRvIII expression was heterogeneous, with both positive and negative populations maintaining the genetic alterations, akin to primary tumors. Furthermore, EGFRvIII defined a hierarchy where EGFRvIII-positive cells gave rise to additional positive and negative cells. Only cells that had recently lost EGFRvIII expression could re-express EGFRvIII, providing an important buffer for maintaining EGFRvIII-positive cell numbers. Epigenetic mechanisms had a role in maintaining heterogeneous EGFRvIII expression. Demethylation induced a 20–60% increase in the percentage of EGFRvIII-positive cells, indicating that some cells could re-express EGFRvIII. Surprisingly, inhibition of histone deacetylation resulted in a 50–80% reduction in EGFRvIII expression. Collectively, this data demonstrates that EGFR amplification and rearrangement are early events in tumorigenesis and EGFRvIII follows a model of hierarchical expression. Furthermore, EGFRvIII expression is restricted by epigenetic mechanisms, suggesting that drugs that modulate the epigenome might be used successfully in glioblastoma tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG . Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 2010; 12: 520–527.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Humphrey PA, Wong AJ, Vogelstein B, Friedman HS, Werner MH, Bigner DD et al. Amplification and expression of the epidermal growth factor receptor gene in human glioma xenografts. Cancer Res 1988; 48: 2231–2238.
Yamazaki H, Fukui Y, Ueyama Y, Tamaoki N, Kawamoto T, Taniguchi S et al. Amplification of the structurally and functionally altered epidermal growth factor receptor gene (c-erbB) in human brain tumors. Mol Cell Biol 1988; 8: 1816–1820.
Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H . Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol 1996; 6: 217–223 discussion 23-24.
Moscatello DK, Holgado-Madruga M, Godwin AK, Ramirez G, Gunn G, Zoltick PW et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res 1995; 55: 5536–5539.
Garcia de Palazzo IE, Adams GP, Sundareshan P, Wong AJ, Testa JR, Bigner DD et al. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res 1993; 53: 3217–3220.
Olapade-Olaopa EO, Moscatello DK, MacKay EH, Horsburgh T, Sandhu DP, Terry TR et al. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer 2000; 82: 186–194.
Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res 1995; 55: 3140–3148.
Tang CK, Gong XQ, Moscatello DK, Wong AJ, Lippman ME . Epidermal growth factor receptor vIII enhances tumorigenicity in human breast cancer. Cancer Res 2000; 60: 3081–3087.
Sugawa N, Ekstrand AJ, James CD, Collins VP . Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA 1990; 87: 8602–8606.
Wong AJ, Ruppert JM, Bigner SH, Grzeschik CH, Humphrey PA, Bigner DS et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA 1992; 89: 2965–2969.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Son MJ, Woolard K, Nam DH, Lee J, Fine HA . SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 2009; 4: 440–452.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760.
Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ . Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci USA 1998; 95: 5724–5729.
Nagane M, Narita Y, Mishima K, Levitzki A, Burgess AW, Cavenee WK et al. Human glioblastoma xenografts overexpressing a tumor-specific mutant epidermal growth factor receptor sensitized to cisplatin by the AG1478 tyrosine kinase inhibitor. J Neurosurg 2001; 95: 472–479.
Holland EC, Hively WP, DePinho RA, Varmus HE . A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev 1998; 12: 3675–3685.
Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O'Rourke DM, Holland EC et al. Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J Neurooncol 2010; 97: 323–337.
Wong AJ, Mitra S, Del Vecchio CA, Skirboll S . Expression of EGFRvIII in brain tumor stem cells [Abstract]. ASCO Annual Meeting Proceedings. J Clin Oncol 2008; 26 (Suppl): 2002.
Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci USA 1990; 87: 4207–4211.
Vogt N, Lefevre SH, Apiou F, Dutrillaux AM, Cor A, Leuraud P et al. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc Natl Acad Sci USA 2004; 101: 11368–11373.
Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M . Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol 2004; 21: 53–56.
Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC et al. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol 2004; 63: 700–707.
Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H . Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol 2004; 14: 131–136.
Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res 1990; 50: 8017–8022.
Jones G, Machado J, Merlo A . Loss of focal adhesion kinase (FAK) inhibits epidermal growth factor receptor-dependent migration and induces aggregation of nh(2)-terminal FAK in the nuclei of apoptotic glioblastoma cells. Cancer Res 2001; 61: 4978–4981.
Frederick L, Eley G, Wang XY, James CD . Analysis of genomic rearrangements associated with EGRFvIII expression suggests involvement of Alu repeat elements. Neuro Oncol 2000; 2: 159–163.
Von Hoff DD, Waddelow T, Forseth B, Davidson K, Scott J, Wahl G . Hydroxyurea accelerates loss of extrachromosomally amplified genes from tumor cells. Cancer Res 1991; 51 (Pt 1): 6273–6279.
Thomas C, Ely G, James CD, Jenkins R, Kastan M, Jedlicka A et al. Glioblastoma-related gene mutations and over-expression of functional epidermal growth factor receptors in SKMG-3 glioma cells. Acta Neuropathol 2001; 101: 605–615.
Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol 2005; 7: 164–176.
Solomon DA, Kim JS, Ressom HW, Sibenaller Z, Ryken T, Jean W et al. Sample type bias in the analysis of cancer genomes. Cancer Res 2009; 69: 5630–5633.
Stockhausen MT, Broholm H, Villingshoj M, Kirchhoff M, Gerdes T, Kristoffersen K et al. Maintenance of EGFR and EGFRvIII expressions in an in vivo and in vitro model of human glioblastoma multiforme. Exp Cell Res 2011; 317: 1513–1526.
Witusik-Perkowska M, Rieske P, Hulas-Bigoszewska K, Zakrzewska M, Stawski R, Kulczycka-Wojdala D et al. Glioblastoma-derived spheroid cultures as an experimental model for analysis of EGFR anomalies. J Neurooncol 2011; 102: 395–407.
Schulte A, Gunther HS, Martens T, Zapf S, Riethdorf S, Wulfing C et al. Glioblastoma stem-like cell lines with either maintenance or loss of high-level EGFR amplification, generated via modulation of ligand concentration. Clin Cancer Res 2012; 18: 1901–1913.
Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell 2010; 6: 141–152.
Chen Z, Clark S, Birkeland M, Sung CM, Lago A, Liu R et al. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) alpha and beta messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett 2002; 188: 127–140.
Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK . Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 2001; 1: 194–202.
Vigushin DM, Ali S, Pace PE, Mirsaidi N, Ito K, Adcock I et al. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res 2001; 7: 971–976.
Yoshida M, Kijima M, Akita M, Beppu T . Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 1990; 265: 17174–17179.
Bialer M, Yagen B . Valproic acid: second generation. Neurotherapeutics 2007; 4: 130–137.
Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 2005; 114: 380–386.
Svechnikova I, Almqvist PM, Ekstrom TJ . HDAC inhibitors effectively induce cell type-specific differentiation in human glioblastoma cell lines of different origin. Int J Oncol 2008; 32: 821–827.
Shabason JE, Tofilon PJ, Camphausen K . Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med 2011; 15: 2735–2744.
Tan J, Cang S, Ma Y, Petrillo RL, Liu D . Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol 2010; 3: 5.
Acknowledgements
We thank Dr Neethan Lobo, Dr Siddhartha Mitra, Dr A Hunter Shain and the Stanford FACS facility for technical advice. Additionally, we thank Dana Bangs and Dr Athena Cherry at the Stanford Cytogenetics Laboratory for their assistance with FISH staining and analysis. This work was supported by the National Defense Science and Engineering Graduate Research Fellowship, the National Science Foundation Graduate Research Fellowship, NIH Grants CA124832, RC2 CA14891, the Lucille Packard Children’s Foundation and a research grant from the National Brain Tumor Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Del Vecchio, C., Giacomini, C., Vogel, H. et al. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene 32, 2670–2681 (2013). https://doi.org/10.1038/onc.2012.280
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.280
Keywords
This article is cited by
-
Intratumoral thrombosis as a histological biomarker for predicting epidermal growth factor receptor alteration and poor prognosis in patients with glioblastomas
Journal of Neuro-Oncology (2023)
-
Preparation and characterization of enzyme-responsive zwitterionic nanoparticles for monoclonal antibody delivery
Frontiers of Materials Science (2023)
-
Glioblastoma mutations alter EGFR dimer structure to prevent ligand bias
Nature (2022)
-
Glioblastoma, an opportunity T cell trafficking could bring for the treatment
Molecular Biology Reports (2022)
-
The University of Pennsylvania glioblastoma (UPenn-GBM) cohort: advanced MRI, clinical, genomics, & radiomics
Scientific Data (2022)