Abstract
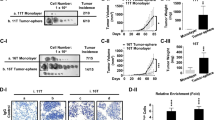
Akt activation is common in progressive thyroid cancer. In breast cancer, Akt1 induces primary cancer growth, but is reported to inhibit metastasis in vivo in several model systems. In contrast, clinical and in vitro studies suggest a metastasis-promoting role for Akt1 in thyroid cancer. The goal of this study was to determine the functional role of Akt1 in thyroid cancer growth and metastatic progression in vivo using thyroid hormone receptor (TR) βPV/PV knock-in (PV) mice, which develop metastatic thyroid cancer. We crossed Akt1−/− and PV mice and compared tumor development, local progression, metastasis and histology in TRβPV/PV/Akt1+/+ (PVPV-Akt1WT) and TRβPV/PV/Akt1−/− (PVPV-Akt1KO) mice. Mice were killed at 3, 6, 9, 12 and 15 months; necropsy was performed and serum thyroid stimulating hormone (TSH) was measured. Thyroid hyperplasia occurred in both groups beginning at 3 months; the thyroid size was greater in the PVPV-Akt1WT mice (P<0.001). In comparison with PVPV-Akt1WT mice, thyroid cancer development was delayed in the PVPV-Akt1KO mice (P=0.003) and the degree of tumor invasiveness was reduced. The PVPV-Akt1WT mice displayed pulmonary metastases at 12 and 15 months of age, by contrast PVPV-Akt1KO mice did not develop distant metastases at 15 months of age. Despite continued expression of Akt2 or Akt3, pAkt levels were decreased and there was evidence of reduced Akt effect on p27 in the PVPV-Akt1KO thyroids. TSH levels were similarly elevated in PV mice regardless of Akt1 expression. In conclusion, thyroid cancer development and progression in TR βPV/PV mice are Akt1-dependent, consistent with a tumor progression-promoting role in this murine thyroid cancer model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chellaiah MA, Biswas RS, Yuen D, Alvarez UM, Hruska KA . (2001). Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J Biol Chem 276: 47434–47444.
Chen J, Tang H, Hay N, Xu J, Ye RD . (2010). Akt isoforms differentially regulate neutrophil functions. Blood 115: 4237–4246.
Chen ML, Xu PZ, Peng XD, Chen WS, Guzman G, Yang X et al. (2006). The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/− mice. Genes Dev 20: 1569–1574.
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T et al. (2001). Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15: 2203–2208.
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH . (2007). Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res 67: 1979–1987.
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw III EB et al. (2001a). Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731.
Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ . (2001b). Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352.
Coulonval K, Vandeput F, Stein RC, Kozma SC, Lamy F, Dumont JE . (2000). Phosphatidylinositol 3-kinase, protein kinase B and ribosomal S6 kinases in the stimulation of thyroid epithelial cell proliferation by cAMP and growth factors in the presence of insulin. Biochem J 348 Part 2: 351–358.
Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP . (2001). Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet 27: 222–224.
Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ . (2009). Akt1 and Akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res 69: 5057–5064.
Dillon RL, Muller WJ . (2010). Distinct biological roles for the akt family in mammary tumor progression. Cancer Res 70: 4260–4264.
Fernandez-Hernando C, Jozsef L, Jenkins D, Di Lorenzo A, Sessa WC . (2009). Absence of Akt1 reduces vascular smooth muscle cell migration and survival and induces features of plaque vulnerability and cardiac dysfunction during atherosclerosis. Arterioscler Thromb Vasc Biol 29: 2033–2040.
Fujita N, Sato S, Katayama K, Tsuruo T . (2002). Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J Biol Chem 277: 28706–28713.
Furuya F, Hanover JA, Cheng S-y . (2006). Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc Natl Acad Sci USA 103: 1780–1785.
Furuya F, Lu C, Willingham MC, Cheng SY . (2007). Inhibition of phosphatidylinositol 3′ kinase delays tumor progression and blocks metastatic spread in a mouse model of thyroid cancer. Carcinogenesis 28: 2451–2458.
Gonzalez E, McGraw TE . (2009). Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA 106: 7004–7009.
Guigon CJ, Fozzatti L, Lu C, Willingham MC, Cheng SY . (2010). Inhibition of mTORC1 signaling reduces tumor growth but does not prevent cancer progression in a mouse model of thyroid cancer. Carcinogenesis 31: 1284–1291.
Héron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA et al. (2006). Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol Cell Biol 26: 8267–8280.
Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ . (2004). Activation of Akt-1 (PKB-α) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res 64: 3171–3178.
Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N et al. (2005). Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol 171: 1023–1034.
Kaneshige M, Kaneshige K, Zhu X, Dace A, Garrett L, Carter TA et al. (2000). Mice with a targeted mutation in the thyroid hormone beta receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc Natl Acad Sci USA 97: 13209–13214.
Kim CF, Vasko VV, Kato Y, Kruhlak M, Saji M, Cheng S-Y et al. (2005). AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology 146: 4456–4463.
Kim CS, Furuya F, Ying H, Kato Y, Hanover JA, Cheng S-Y . (2007). Gelsolin: a novel thyroid hormone receptor-β interacting protein that modulates tumor progression in a mouse model of follicular thyroid cancer. Endocrinology 148: 1306–1312.
Kim CS, Zhu X . (2009). Lessons from mouse models of thyroid cancer. Thyroid 19: 1317–1331.
Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP . (2001). Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22: 631–630.
Larrea MD, Hong F, Wander SA, da Silva TG, Helfman D, Lannigan D et al. (2009). RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci USA 106: 9268–9273.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K et al. (2002). PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 8: 1153–1160.
Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA et al. (2006). Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proc Natl Acad Sci USA 103: 4134–4139.
Lu C, Zhao L, Ying H, Willingham MC, Cheng S-Y . (2010). Growth activation alone is not sufficient to cause metastatic thyroid cancer in a mouse model of follicular thyroid carcinoma. Endocrinology 151: 1929–1939.
McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF . (2003). Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol 23: 216–228.
Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH . (2006). Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal 18: 2262–2271.
Mielnicki LM, Ying AM, Head KL, Asch HL, Asch BB . (1999). Epigenetic regulation of gelsolin expression in human breast cancer cells. Exp Cell Res 249: 161–176.
Ni XG, Zhou L, Wang GQ, Liu SM, Bai XF, Liu F et al. (2008). The ubiquitin-proteasome pathway mediates gelsolin protein downregulation in pancreatic cancer. Mol Med 14: 582–589.
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM et al. (1999). Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96: 1563–1568.
Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S . (1999). Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9: 1265–1271.
Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M et al. (2009). Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69: 4885–4893.
Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H et al. (2001). Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 61: 6105–6111.
Saito J, Kohn AD, Roth RA, Noguchi Y, Tatsumo I, Hirai A et al. (2001). Regulation of FRTL-5 thyroid cell growth by phosphatidylinositol (OH) 3 kinase-dependent Akt-mediated signaling. Thyroid 11: 339–351.
Saji M, Vasko V, Kada F, Allbritton EH, Burman KD, Ringel MD . (2005). Akt1 contains a functional leucine-rich nuclear export sequence. Biochem Biophys Res Commun 332: 167–173.
Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J et al. (2002). PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat Med 8: 1145–1152.
Stambolic V, Woodgett JR . (2006). Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol 16: 461–466.
Suzuki H, Willingham MC, Cheng SY . (2002). Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12: 963–969.
Tanaka H, Shirkoohi R, Nakagawa K, Qiao H, Fujita H, Okada F et al. (2006). siRNA gelsolin knockdown induces epithelial-mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int J Cancer 118: 1680–1691.
Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N et al. (2009). Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461: 1084–1091.
Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V et al. (2004). Akt activation and localization correlate with tumor invasion and oncogene expression in thyroid cancer. J Mol Genet 41: 161–170.
Viglietto G, Motti ML, Bruni P, Melillo RM, D′Alessio A, Califano D et al. (2002). Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med 8: 1136–1144.
Wu FY, Wang SE, Sanders ME, Shin I, Rojo F, Baselga J et al. (2006). Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res 66: 2162–2172.
Ying H, Suzuki H, Furumoto H, Walker R, Meltzer P, Willingham MC et al. (2003). Alterations in genomic profiles during tumor progression in a mouse model of follicular thyroid carcinoma. Carcinogenesis 24: 1467–1479.
Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A . (2005). Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell 20: 539–550.
Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J . (2006). Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem 281: 36443–36453.
Acknowledgements
Funding for the work is from NIH grants (P01CA124570 and R01CA102572) to MDR. We appreciate the technical assistance of Michael Ostrowski, John Thompson and Jun Liu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Ringel has received honoraria for advisory boards from Veracyte and Astra Zeneca. This was not related to the work presented in the manuscript. He has received funding from NIH for this work. M Saji, K Narahara, S McCarty, V Vasko, K La Perle, K Porter, D Jarjoura, C Lu and S-Y Cheng all declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Saji, M., Narahara, K., McCarty, S. et al. Akt1 deficiency delays tumor progression, vascular invasion, and distant metastasis in a murine model of thyroid cancer. Oncogene 30, 4307–4315 (2011). https://doi.org/10.1038/onc.2011.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.136
Keywords
This article is cited by
-
Pathogenesis of cancers derived from thyroid follicular cells
Nature Reviews Cancer (2023)
-
Targeting Akt in cancer for precision therapy
Journal of Hematology & Oncology (2021)
-
Transcriptome analysis discloses dysregulated genes in normal appearing tumor-adjacent thyroid tissues from patients with papillary thyroid carcinoma
Scientific Reports (2021)
-
Akt isoform-specific effects on thyroid cancer development and progression in a murine thyroid cancer model
Scientific Reports (2020)
-
Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes
Oncogene (2016)