Abstract
Polarized epithelia form by oriented cell divisions in which the mitotic spindle aligns parallel to the epithelial plane. To orient the mitotic spindle, cortical cues trigger the recruitment of NuMA–dynein–based motors, which pull on astral microtubules via the protein LGN. We demonstrate that the junctional protein Afadin is required for spindle orientation and correct epithelial morphogenesis of Caco-2 cysts. Molecularly, Afadin binds directly and concomitantly to F-actin and to LGN. We determined the crystallographic structure of human Afadin in complex with LGN and show that it resembles the LGN–NuMA complex. In mitosis, Afadin is necessary for cortical accumulation of LGN and NuMA above the spindle poles, in an F-actin–dependent manner. Collectively, our results depict Afadin as a molecular hub governing the enrichment of LGN and NuMA at the cortex. To our knowledge, Afadin is the first-described mechanical anchor between dynein and cortical F-actin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morin, X. & Bellaïche, Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell 21, 102–119 (2011).
Lancaster, M.A. & Knoblich, J.A. Spindle orientation in mammalian cerebral cortical development. Curr. Opin. Neurobiol. 22, 737–746 (2012).
Williams, S.E., Beronja, S., Pasolli, H.A. & Fuchs, E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470, 353–358 (2011).
Pease, J.C. & Tirnauer, J.S. Mitotic spindle misorientation in cancer: out of alignment and into the fire. J. Cell Sci. 124, 1007–1016 (2011).
Gonzalez, C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat. Rev. Genet. 8, 462–472 (2007).
Knoblich, J.A. Asymmetric cell division: recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 11, 849–860 (2010).
Noatynska, A., Gotta, M. & Meraldi, P. Mitotic spindle (DIS)orientation and DISease: cause or consequence? J. Cell Biol. 199, 1025–1035 (2012).
Kotak, S. & Gönczy, P. Mechanisms of spindle positioning: cortical force generators in the limelight. Curr. Opin. Cell Biol. 25, 741–748 (2013).
Du, Q. & Macara, I.G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004).
Kotak, S., Busso, C. & Gönczy, P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 199, 97–110 (2012).
Saadaoui, M. et al. Dlg1 controls planar spindle orientation in the neuroepithelium through direct interaction with LGN. J. Cell Biol. 206, 707–717 (2014).
Bergstralh, D.T., Lovegrove, H.E. & St Johnston, D. Discs large links spindle orientation to apical-basal polarity in Drosophila epithelia. Curr. Biol. 23, 1707–1712 (2013).
Nakajima, Y., Meyer, E.J., Kroesen, A., McKinney, S.A. & Gibson, M.C. Epithelial junctions maintain tissue architecture by directing planar spindle orientation. Nature 500, 359–362 (2013).
Johnston, C.A., Hirono, K., Prehoda, K.E. & Doe, C.Q. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138, 1150–1163 (2009).
Zhu, J. et al. Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 30, 4986–4997 (2011).
Johnston, C.A., Doe, C.Q. & Prehoda, K.E. Structure of an enzyme-derived phosphoprotein recognition domain. PLoS One 7, e36014 (2012).
Culurgioni, S., Alfieri, A., Pendolino, V., Laddomada, F. & Mapelli, M. Inscuteable and NuMA proteins bind competitively to Leu-Gly-Asn repeat-enriched protein (LGN) during asymmetric cell divisions. Proc. Natl. Acad. Sci. USA 108, 20998–21003 (2011).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Speicher, S., Fischer, A., Knoblich, J. & Carmena, A. The PDZ protein Canoe regulates the asymmetric division of Drosophila neuroblasts and muscle progenitors. Curr. Biol. 18, 831–837 (2008).
Carmena, A., Makarova, A. & Speicher, S. The Rap1-Rgl-Ral signaling network regulates neuroblast cortical polarity and spindle orientation. J. Cell Biol. 195, 553–562 (2011).
Wee, B., Johnston, C.A., Prehoda, K.E. & Doe, C.Q. Canoe binds RanGTP to promote Pins(TPR)/Mud-mediated spindle orientation. J. Cell Biol. 195, 369–376 (2011).
Mandai, K. et al. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139, 517–528 (1997).
Niessen, C.M. & Gottardi, C.J. Molecular components of the adherens junction. Biochim. Biophys. Acta 1778, 562–571 (2008).
Ooshio, T. et al. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J. Cell Sci. 120, 2352–2365 (2007).
Zhadanov, A.B. et al. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr. Biol. 9, 880–888 (1999).
Yamamoto, H. et al. Genetic deletion of afadin causes hydrocephalus by destruction of adherens junctions in radial glial and ependymal cells in the midbrain. PLoS One 8, e80356 (2013).
Yang, Z. et al. De novo lumen formation and elongation in the developing nephron: a central role for afadin in apical polarity. Development 140, 1774–1784 (2013).
Tawa, H. et al. Role of afadin in vascular endothelial growth factor- and sphingosine 1-phosphate-induced angiogenesis. Circ. Res. 106, 1731–1742 (2010).
Tanaka-Okamoto, M. et al. Genetic ablation of afadin causes mislocalization and deformation of Paneth cells in the mouse small intestinal epithelium. PLoS One 9, e110549 (2014).
Kobayashi, R. et al. s-Afadin binds more preferentially to the cell adhesion molecules nectins than l-afadin. Genes Cells 19, 853–863 (2014).
Johnston, C.A. et al. Formin-mediated actin polymerization cooperates with Mushroom body defect (Mud)-Dynein during Frizzled-Dishevelled spindle orientation. J. Cell Sci. 126, 4436–4444 (2013).
Lorger, M. & Moelling, K. Regulation of epithelial wound closure and intercellular adhesion by interaction of AF6 with actin cytoskeleton. J. Cell Sci. 119, 3385–3398 (2006).
Théry, M., Jiménez-Dalmaroni, A., Racine, V., Bornens, M. & Jülicher, F. Experimental and theoretical study of mitotic spindle orientation. Nature 447, 493–496 (2007).
Lancaster, O.M. & Baum, B. Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 34, 109–115 (2014).
Seldin, L., Poulson, N.D., Foote, H.P. & Lechler, T. NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol. Biol. Cell 24, 3651–3662 (2013).
Kiyomitsu, T. & Cheeseman, I.M. Cortical dynein and asymmetric membrane elongation coordinately position the spindle in anaphase. Cell 154, 391–402 (2013).
Zheng, Z. et al. Evidence for dynein and astral microtubule-mediated cortical release and transport of Gαi/LGN/NuMA complex in mitotic cells. Mol. Biol. Cell 24, 901–913 (2013).
Zhu, J. et al. LGN/mInsc and LGN/NuMA complex structures suggest distinct functions in asymmetric cell division for the Par3/mInsc/LGN and Gαi/LGN/NuMA pathways. Mol. Cell 43, 418–431 (2011).
Yuzawa, S., Kamakura, S., Iwakiri, Y., Hayase, J. & Sumimoto, H. Structural basis for interaction between the conserved cell polarity proteins Inscuteable and Leu-Gly-Asn repeat-enriched protein (LGN). Proc. Natl. Acad. Sci. USA 108, 19210–19215 (2011).
Mapelli, M. & Gonzalez, C. On the inscrutable role of Inscuteable: structural basis and functional implications for the competitive binding of NuMA and Inscuteable to LGN. Open Biol. 2, 120102 (2012).
Kotak, S., Busso, C. & Gönczy, P. NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function. EMBO J. 32, 2517–2529 (2013).
Indra, I., Troyanovsky, R. & Troyanovsky, S.M. Afadin controls cadherin cluster stability using clathrin-independent mechanism. Tissue Barriers 2, e28687 (2014).
Zheng, Z. et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 189, 275–288 (2010).
Pan, Z. et al. Structural and biochemical characterization of the interaction between LGN and Frmpd1. J. Mol. Biol. 425, 1039–1049 (2013).
Zheng, Z., Wan, Q., Meixiong, G. & Du, Q. Cell cycle-regulated membrane binding of NuMA contributes to efficient anaphase chromosome separation. Mol. Biol. Cell 25, 606–619 (2014).
Kotak, S. & Gönczy, P. NuMA phosphorylation dictates dynein-dependent spindle positioning. Cell Cycle 13, 177–178 (2014).
Kiyomitsu, T. & Cheeseman, I.M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat. Cell Biol. 14, 311–317 (2012).
Ségalen, M. et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev. Cell 19, 740–752 (2010).
Machicoane, M. et al. SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation. J. Cell Biol. 205, 791–799 (2014).
Jaffe, A.B., Kaji, N., Durgan, J. & Hall, A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J. Cell Biol. 183, 625–633 (2008).
Ooshio, T. et al. Involvement of LMO7 in the association of two cell-cell adhesion molecules, nectin and E-cadherin, through afadin and alpha-actinin in epithelial cells. J. Biol. Chem. 279, 31365–31373 (2004).
Williams, S.E., Ratliff, L.A., Postiglione, M.P., Knoblich, J.A. & Fuchs, E. Par3-mInsc and Gαi3 cooperate to promote oriented epidermal cell divisions through LGN. Nat. Cell Biol. 16, 758–769 (2014).
Elias, S. et al. Huntingtin regulates mammary stem cell division and differentiation. Stem Cell Reports 2, 491–506 (2014).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Carlier, M.F., Criquet, P., Pantaloni, D. & Korn, E.D. Interaction of cytochalasin D with actin filaments in the presence of ADP and ATP. J. Biol. Chem. 261, 2041–2050 (1986).
Scita, G. et al. An effector region in Eps8 is responsible for the activation of the Rac-specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J. Cell Biol. 154, 1031–1044 (2001).
Acknowledgements
We are grateful to S. Pasqualato and V. Cecatiello of the Crystallography Unit of the European Institute of Oncology for technical support. We thank E. Martini and D. Parazzoli of the Cogentech Facility for help with image analysis. We thank scientists at the X06DA beamline at the Swiss Light Source and the ID23-2 beamline at the European Synchrotron Radiation Facility for valuable help with data collection. We are grateful to all members of the Mapelli laboratory, F. Villa, G. Scita, A. Disanza, S. Santaguida and A. De Antoni for scientific discussions, and to A. Musacchio for careful reading of the manuscript. We thank Y. Takai (Kobe University Graduate School of Medicine, Japan) for sharing the rat Afadin cDNA. This work was supported by grants from the Italian Association for Cancer Research (AIRC) to M.M., the Italian Ministry of Health to M.M. and BioStruct-X (FP7/2007-2013, grant agreement 283570).
Author information
Authors and Affiliations
Contributions
M.C. conducted the biochemical and structural biology experiments, and S.G. performed cell biology experiments and analysis. A.A. designed Afadin mutants, L.P. helped with the cell biology, and S.B. provided purified actin. M.M. supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
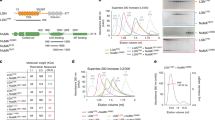
Supplementary Figure 1 Characterization of Afadin-, LGN- and NuMA-specific antibodies, and spindle angle analysis in LGN-depleted and NuMA-depleted HeLa cells.
(a) Scheme of human Afadin (also known as AF-6 or MLLT4) gene, and its splice variants. The AF-6 gene is located on human chromosome 6, and consists of 32 exons. Alternative splicing produces six transcripts differing in their C-terminal region. Human Afadin isoforms 1 and 6 stop at exons 28 and 29 respectively, and are similar to short variant of rat Afadin (also known as s-Afadin), which was reported to be unable to bind to F-actin. Human Afadin isoforms 2, 3, 4 and 5 are similar to long rat Afadin (l-Afadin), which binds to F-actin. Isoforms 4 and 5 differ for the presence of additional 11 residues between exon 28 and 29. The LGN-binding site of Afadin characterized in this study is coded by exon 30 (highlighted in orange), and is present in all human long isoforms except isoform-3. (b) Specificity of the Afadin antibody. Confocal sections of mitotic HeLa cells expressing a control shRNA and shRNA targeting Afadin (Afadin shRNA-2), fixed and stained for endogenous Afadin. Afadin staining was lost in Afadin shRNA-2 expressing cells. The scale bar corresponds to 5 μm. (c) Immunoblot analysis of mitotic lysates of HeLa cells transiently interfered for LGN (left) or stably depleted of NuMA (left) showing the efficiency of the protein depletions. (d) Representative confocal z-sections of mitotic HeLa cells depleted for LGN (left) or NuMA (right), with the corresponding controls. LGN-depleted cells are stained with NuMA (green) and DAPI (blue), while NuMA-ablated cells are visualized with γ-tubulin (dark yellow) and DAPI (blue). The plane of the coverslip is visible as a white line. (e) Quantification of mitotic spindle alignment performed as in Fig. 1 (with means ± SEM), showing mean angular tilts of about 14.9° for NuMA-depleted cells, and 12.5° for LGN-ablated cells. **** indicates a statistical difference of P < 0.0001 by Mann-Whitney test between interfered and control cells from three independent experiments with n > 50. Scale bars correspond to 5 μM.
Supplementary Figure 2 Canoe (Afadin) binds directly to Pins (LGN) and competes with Mud (NuMA).
SEC analysis and corresponding Coomassie-blue stained SDS-PAGE of the competitive interactions between the C-terminal portion of Drosophila Canoe (encompassing residues 1755-2051), Pins(LGN)TPR (spanning residues 25-406) and Mud1895-2094. At equimolar concentration, Canoe(Afadin)Cter enters a stoichiometric complex with Pins(LGN)TPR eluting in fractions 3-6 (black trace). Addition to the mix of equimolar amounts of Mud1895-2094 results in a complex of Mud1895-2094–Pins(LGN)TPR eluting slightly earlier than Canoe(Afadin)Cter in isolation (fractions 4-7). This analysis demonstrates that Canoe(Afadin) and Mud(NuMA) are mutually exclusive interactors of Pins(LGN)TPR, with Mud(NuMA) displaying higher affinity.
Supplementary Figure 3 Sequence alignment of the C terminus of Afadin long isoforms.
Afadin residues are colored based on the conservation calculated on the alignment of seven orthologues of Homo sapiens, Rattus norvegicus, Falco cherrug, Chelonia mydas, Xenopus laevis, Danio rerio and Drosophila melanogaster. The orange line highlights the Afadin fragment directly in contact with the LGNTPR domain according to the crystallographic structure, with the Phe1730AF and Glu1735AF labeled as red circles. The F-actin binding region of Afadin determined experimentally (Supplementary Figure 4b) is upstream of the LGN-binding stretch, and is predicted to adopt a helical conformation, as depicted in light gray (secondary structure prediction was performed using the server https://www.predictprotein.org/).
Supplementary Figure 4 Biochemical characterization of the interaction between AfadinCter and actin.
(a) Afadin does not bind to globular actin (G-actin). SEC analysis and corresponding SDS-PAGE showing that at 150 μM concentration of both species AfadinCter and monomeric G-actin do not form a complex. The elution profiles of AfadinCter and G-actin are also shown as references for the individual runs. Despite having similar molecular weight, AfadinCter elutes near the globular 158 kDa marker (lanes 3-6 of the SDS-PAGE), whereas G-actin elutes in the later fractions 7-10. (b) Afadin binds to F-actin through a region lying upstream to the LGN binding site. High-speed cosedimentation assay of two complementary fragments of AfadinCter (residues 1514-1824) with F-actin, showing that the actin-binding domain of human Afadin spans residues 1514-1682, upstream of the LGN-binding domain (colored in purple in the top scheme). Supernatant and pellet fractions of an analogous high-speed sedimentation experiment performed in the absence of F-actin are shown as a control of cosedimentation specificity.
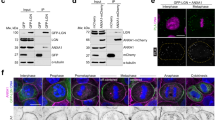
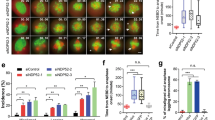
Supplementary Figure 5 Analysis of spindle motors in Afadin-depleted HeLa anaphases, localization of Afadin rescue constructs and NuMA rescue experiments.
(a) Ablation of Afadin in HeLa cells impairs cortical localization of LGN, NuMA and Dynactin in anaphase. Confocal sections of control shRNA (top) and Afadin shRNA-2 expressing HeLa cells (bottom) in anaphase stained for LGN, NuMA, and p150Glued. Chromosomes are visualized with DAPI (blue). (b) Immunostaining of endogenous Afadin in mitotic HeLa cells interfered for LGN (left) or NuMA (right) with the corresponding controls. DNA is stained with DAPI. Quantification of cortical signals revealed that the cortical accumulation of Afadin is not altered by loss of LGN nor NuMA. (c-d) mCherry-Afadin rescue constructs. Immunoblot and immunostaining of HeLa cells transiently transfected with mCherry-Afadin, mCherry-Afadin-ΔLGN, or mCherry-Afadin-ΔACTIN showing the expression levels and the cortical localization of the shRNA-2 resistant rescue constructs. (e) Quantification of cortical mCherry-Afadin constructs analyzed in (d) showing that the F-actin binding domain of Afadin is required for the correct localization of the protein at the cortex. **** indicates P < 0.0001 by Kruskal-Wallis test from three independent experiments with n > 40. (f) Rescue experiment of cortical NuMA in HeLa cells stably depleted of Afadin, and transfected with shRNA-2 resistant mCherry-tagged rat Afadin wild-type, Afadin-ΔLGN, or Afadin-ΔACTIN. Quantification of cortical NuMA signals indicates that only Afadin wild-type rescues the cortical localization of NuMA in metaphase. The bottom panels show the mCherry-signal of the transfected cells. Under the conditions of methanol fixation used to visualize cortical NuMA, the cortical mCherry signal of all the rescue constructs is lost. **** indicates P < 0.0001 by Kruskal-Wallis test from three independent experiments with n > 32. Scale bars correspond to 5 μm.
Supplementary Figure 6 Distribution of cortical F-actin in metaphases of Caco-2 cells, either wild type or lacking Afadin in three-dimensional cysts.
Cysts of Caco-2 cells wild-type or stably interfered for Afadin stained with DAPI (blue) and TRITC-conjugated Phalloidin (red). Confocal sections of the equatorial region of the cysts show that in mitotic cells (indicated by grey arrows) cortical F-actin distributes uniformly all around the plasma membrane regardless of the presence of Afadin.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1188 kb)
Supplementary Data Set 1
Uncropped gels (PDF 441 kb)
Rights and permissions
About this article
Cite this article
Carminati, M., Gallini, S., Pirovano, L. et al. Concomitant binding of Afadin to LGN and F-actin directs planar spindle orientation. Nat Struct Mol Biol 23, 155–163 (2016). https://doi.org/10.1038/nsmb.3152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3152
This article is cited by
-
Annexin A1 is a polarity cue that directs mitotic spindle orientation during mammalian epithelial morphogenesis
Nature Communications (2023)
-
Spindle positioning and its impact on vertebrate tissue architecture and cell fate
Nature Reviews Molecular Cell Biology (2021)
-
Structural and functional insight into the effect of AFF4 dimerization on activation of HIV-1 proviral transcription
Cell Discovery (2020)
-
mTOR and S6K1 drive polycystic kidney by the control of Afadin-dependent oriented cell division
Nature Communications (2020)
-
Hexameric NuMA:LGN structures promote multivalent interactions required for planar epithelial divisions
Nature Communications (2019)