Abstract
Toll-like receptors (TLRs) have crucial roles in innate immunity, functioning as pattern-recognition receptors. TLR13 recognizes a conserved sequence from bacterial 23S rRNA and then triggers an immune response. Here we report the crystal structure of the mouse TLR13 ectodomain bound by a 13-nt single-stranded (ss) RNA derived from 23S rRNA. The ssRNA induces TLR13 dimerization but assumes a stem-loop-like structure that is completely different from that in the bacterial ribosome but nevertheless is crucial for TLR13 recognition. Most of the RNA nucleotides are splayed out to make base-specific contacts with the concave surface of TLR13, and RNA-specific interactions are important to allow TLR13 to distinguish RNA from DNA. Interestingly, a viral-derived 16-nt ssRNA predicted to form a similar stem-loop-like structure also induces TLR13 activation. Together, our results reveal the structural mechanism of TLR13's sequence- and conformation-specific recognition of ssRNA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Aderem, A. & Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 (2000).
Kang, J.Y. & Lee, J.O. Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 80, 917–941 (2011).
Botos, I., Segal, D.M. & Davies, D.R. The structural biology of Toll-like receptors. Structure 19, 447–459 (2011).
Blasius, A.L. & Beutler, B. Intracellular toll-like receptors. Immunity 32, 305–315 (2010).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Diebold, S.S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Oldenburg, M. et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337, 1111–1115 (2012).
Li, X.D. & Chen, Z.J. Sequence specific detection of bacterial 23S ribosomal RNA by TLR13. eLife 1, e00102 (2012).
Shi, Z. et al. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 286, 4517–4524 (2011).
Jin, M.S. et al. Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 (2007).
Liu, L. et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320, 379–381 (2008).
Park, B.S. et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 (2009).
Kang, J.Y. et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31, 873–884 (2009).
Yoon, S.I. et al. Structural basis of TLR5-flagellin recognition and signaling. Science 335, 859–864 (2012).
Tanji, H., Ohto, U., Shibata, T., Miyake, K. & Shimizu, T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science 339, 1426–1429 (2013).
Ohto, U. et al. Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9. Nature 520, 702–705 (2015).
Dunkle, J.A., Xiong, L., Mankin, A.S. & Cate, J.H. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl. Acad. Sci. USA 107, 17152–17157 (2010).
Thapar, R., Denmon, A.P. & Nikonowicz, E.P. Recognition modes of RNA tetraloops and tetraloop-like motifs by RNA-binding proteins. Wiley Interdiscip. Rev. RNA 5, 49–67 (2014).
Du, Z. et al. Crystal structure of the first KH domain of human poly(C)-binding protein-2 in complex with a C-rich strand of human telomeric DNA at 1.7 A. J. Biol. Chem. 280, 38823–38830 (2005).
Weiss, M.S., Brandl, M., Suhnel, J., Pal, D. & Hilgenfeld, R. More hydrogen bonds for the (structural) biologist. Trends Biochem. Sci. 26, 521–523 (2001).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Radermacher, M., Wagenknecht, T., Verschoor, A. & Frank, J. Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli. J. Microsc. 146, 113–136 (1987).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
van Heel, M. & Keegstra, W. IMAGIC: a fast, flexible and friendly image analysis software system. Ultramicroscopy 7, 113–129 (1981).
Frank, J. et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 116, 190–199 (1996).
Shaikh, T.R. et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 3, 1941–1974 (2008).
Scheres, S.H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Terwilliger, T.C. Using prime-and-switch phasing to reduce model bias in molecular replacement. Acta Crystallogr. D Biol. Crystallogr. 60, 2144–2149 (2004).
Terwilliger, T.C. Automated main-chain model building by template matching and iterative fragment extension. Acta Crystallogr. D Biol. Crystallogr. 59, 38–44 (2003).
Terwilliger, T.C. Automated side-chain model building and sequence assignment by template matching. Acta Crystallogr. D Biol. Crystallogr. 59, 45–49 (2003).
Read, R.J. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986).
Cowtan, K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 (2006).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Laskowski, R.A., MacArthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Vriend, G. WHAT IF: a molecular modeling and drug design program. J. Mol. Graph. 8, 52–56, 29 (1990).
Jerabek-Willemsen, M., Wienken, C.J., Braun, D., Baaske, P. & Duhr, S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev. Technol. 9, 342–353 (2011).
Acknowledgements
We thank F. Yu and J. He (both at the Shanghai Synchrotron Radiation Facility (SSRF) BL17U1) and Y. Xu and T. Yang (both at the Tsinghua University Branch of the China National Center for Protein Sciences Beijing) for data collection and computational support; X. Li for technical instructions for using the K2-Summit camera; and L. Jin and L. Liu (both at Peking University) for instructions for microscale thermophoresis. We acknowledge computational-facility support for the 'Explorer 100' cluster system of the Tsinghua National Laboratory for Information Science and Technology. This research was funded by the Chinese Ministry of Science and Technology (2014CB910101 to J. Chai; 2010CB912401 and 2012CB917303 to H.-W.W.; and 2011CB911102 and 2015CB910104 to Jiawei Wang).
Author information
Authors and Affiliations
Contributions
J. Chai, H.-W.W., Jiawei Wang and Z.H. designed the experiments, which were performed by W.S., Jia Wang, H.Z., W.W., S.F. and J. Chang. Data were analyzed by J. Chai, H.-W.W., Jiawei Wang, Z.H., W.S., Y.Z., B.X., D.Z. and Jia Wang. J. Chai, H.-W.W. and Jiawei Wang wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
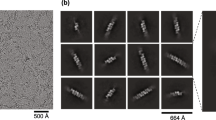
Supplementary Figure 1 Negative-staining EM and cryo-EM of the TLR13LRR–ssRNA complex.
(a) A tilt-pair of EM micrographs of negatively stained TLR13LRR-ssRNA13 complex specimen at 0° and 50° tilt angles respectively. The corresponding tilting pair images from same molecules were marked in the same color boxes within each micrograph. (b) 3D reconstruction of the negatively stained specimen using the RCT method followed by a refinement (10,272 particles from 66 micrographs were used). This model served as the initial model for 3D classification of the CryoEM data in RELION. (c) 3D map of TLR13LRR-25nt ssRNA complex with C2 symmetry enforced during 3D auto-refinement. (d) Gold-standard Fourier shell correlation curves of the map (a) and (Fig. 1c).
Supplementary Figure 2 Molecular replacement with cryo-EM 3D reconstruction for determining the crystal structure of TLR13–ssRNA13.
(a) Plot of figure-of-merit weighted phase errors (wMPE) calculated relative to the subsequently refined structure versus resolution for the various steps of phase improvement, and maps of the same region of the structure calculated with (b) phases from Cryo-EM potential map based molecular replacement solution (MapCC 0.33). Electron density (MapCC 0.55) after 1 cycle (c) and 5 cycles (d) of density modification (MapCC 0.77). The backbone of the final model is displayed in (b), (c) and (d).
Supplementary Figure 3 Mutagenesis analysis of TLR13–ssRNA and homodimeric interfaces.
Left panel: the gel filtration chromatograms of TTLR13LRR wild type (WT) and mutations in response to the ssRNA13. Right panel: Coomassie blue staining of the peak fractions shown on the top following SDS-PAGE. Original gels can be found in (Supplementary Data Set 1).
Supplementary Figure 4 ssDNA with the same sequence as ssRNA13 is unable to induce TLR13 dimerization.
(a) Left panel: superposition of the gel filtration chromatograms of TLR13 LRR domain in the absence (black) or presence (blue) of ssDNA13 (5’ACGGAAAGACCCC3’). The vertical and horizontal axes represent ultraviolet absorbance (λ=280 nm) and elution volume (ml), respectively. Right panel: Coomassie blue staining of the peak fractions shown on the top following SDS-PAGE. “M”: molecular weight marker. Original gels can be found in (Supplementary Data Set 1). (b) Quantification analysis of ssRNA13- or ssDNA13-TLR13 interaction using Microscale Thermophresis (MST). Data points indicate the fluorescence [‰] generated by ssRNA13 or ssDNA13 binding TLR13LRR, and curves indicate the calculated fits. Error bars represent standard error of 3 independent measurements. The assay showed that ssRNA13 bound to TLR13LRR with a dissociation constant ~15 nM.
Supplementary Figure 5 A viral-derived ssRNA induces TLR13 dimerization in solution.
ssRNA-VSV16 induces TLR13 dimerization in gel filtration. Left panel: gel filtration chromatograms of TLR13LRR in the presence or absence of ssRNA-VSV16. Right panel: Coomassie blue staining of the peak fractions shown on the left following SDS-PAGE. Original gels can be found in (Supplementary Data Set 1).
Supplementary Figure 6 Sequence alignment of ssRNA13 from different bacterial strains and position of ssRNA13 in the bacterial ribosome.
(a) In each sequence, the two nucleotides highlighted in red corresponds to the Watson-Crick base pair observed in the structure of E coli. ssRNA13 bound by TLR13. (b) Left panel: the 23S ribosome structure (PDB code: 4V7U)20 shown in surface (transparent). ssRNA13 in the ribosome is shown cartoon and labeled. Right panel: a close-up view of ssRNA13 highlighted in the left panel.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1074 kb)
Supplementary Data Set 1
Original gels (PDF 375 kb)
Rights and permissions
About this article
Cite this article
Song, W., Wang, J., Han, Z. et al. Structural basis for specific recognition of single-stranded RNA by Toll-like receptor 13. Nat Struct Mol Biol 22, 782–787 (2015). https://doi.org/10.1038/nsmb.3080
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3080