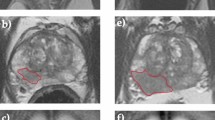
Abstract
A significant amount of research has focused on the role of diffusion-weighted MRI (DW-MRI) in the management of patients with prostate cancer. Although uncertainties remain, a clearer picture of where this technique fits into clinical practice is now available. A combination of DW-MRI and T2-weighted MRI (T2W-MRI) demonstrates improved accuracy for lesion detection and localization compared with T2W-MRI alone, and has been suggested as a tool to guide tissue biopsy. DW-MRI could also have roles in active surveillance, evaluating treatment efficacy, and predicting disease recurrence. Furthermore, DW-MRI offers the exciting possibility of gathering information about tumor characteristics and aggressiveness in a noninvasive manner. Validation in large prospective multicenter trials is critical if this technique is to be integrated into current management algorithms for prostate cancer.
Key Points
-
Adding diffusion-weighted MRI (DW-MRI) to traditional T2-weighted imaging improves the accuracy of prostate cancer detection and could be a useful tool before rebiopsy to increase yield and accuracy
-
During prostate cancer staging, DW-MRI can enhance the detection of invasion into seminal vesicles and the bladder neck, as well as regional lymph node invasion and distant metastases to bone
-
DW-MRI shows promise as a noninvasive biomarker of tumor characteristics, such as Gleason grade, cellular differentiation, tumor cell density, and tumor aggressiveness
-
DW-MRI has potential roles in the management of patients under active surveillance, and in monitoring treatment efficacy and disease recurrence in the follow-up period after treatment
-
Advancements in the processing and analysis of data obtained from DW-MRI might lead to improved characterization of tumor lesions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A. et al. Global cancer statistics. CA Cancer J. Clin. 61, 69–90 (2011).
Hricak, H., Choyke, P., Eberhardt, S., Leibel, S. & Scardino, P. Imaging prostate cancer: a multidisciplinary perspective. Radiology 243, 28–53 (2007).
Patterson, D., Padhani, A. & Collins, D. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat. Clin. Pract. Oncol. 5, 220–233 (2008).
Ogura, A., Hayakawa, K., Miyati, T. & Maeda, F. Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging. Eur. J. Radiol. 77, 185–189 (2011).
Gibbs, P., Pickles, M. & Turnbull, L. Repeatability of echo-planar-based diffusion measurements of the human prostate at 3 T. Magn. Reson. Imaging 25, 1423–1429 (2007).
Braithwaite, A., Dale, B., Boll, D. & Merkle, E. Short and midterm reproducibility of apparent diffusion coefficient measurements at 3.0-T diffusion-weighted imaging of the abdomen. Radiology 250, 459–465 (2009).
Rosenkrantz, A. B., Oei, M., Babb, J. S., Niver, B. E. & Taouli, B. Diffusion-weighted imaging of the abdomen at 3.0 Tesla: image quality and apparent diffusion coefficient reproducibility compared with 1.5 Tesla. J. Magn. Reson. Imaging 33, 128–135 (2011).
Padhani, A. et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11, 102–125 (2009).
Katahira, K. et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur. Radiol. 21, 188–196 (2011).
Rosenkrantz, A. B. et al. Prostate cancer: comparison of tumor visibility on trace diffusion-weighted images and the apparent diffusion coefficient map. AJR Am. J. Roentgenol. 196, 123–129 (2011).
Dehmeshki, J. et al. Analysis of MTR histograms in multiple sclerosis using principal components and multiple discriminant analysis. Magn. Reson. Med. 46, 600–609 (2001).
Tozer, D. et al. Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed. 20, 49–57 (2007).
Nowosielski, M. et al. ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53, 291–302 (2011).
Kyriazi, S. et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging and value of histogram analysis of apparent diffusion coefficients. Radiology 261, 182–192 (2011).
Canuto, H. C. et al. Characterization of image heterogeneity using 2D Minkowski functionals increases the sensitivity of detection of a targeted MRI contrast agent. Magn. Reson. Med. 61, 1218–1224 (2009).
Walker-Samuel, S., Orton, M., Boult, J. K. & Robinson, S. P. Improving apparent diffusion coefficient estimates and elucidating tumor heterogeneity using Bayesian adaptive smoothing. Magn. Reson. Med. 65, 438–447 (2011).
Koh, D. M., Collins, D. J. & Orton, M. R. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am. J. Roentgenol. 196, 1351–1361 (2011).
Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53, 1432–1440 (2005).
Kattan, M. W. Judging new markers by their ability to improve predictive accuracy. J. Natl Cancer Inst. 95, 634–635 (2003).
Eggener, S. et al. Focal therapy for prostate cancer: possibilities and limitations. Eur. Urol. 58, 57–64 (2010).
Rosenkrantz, A. B., Scionti, S. M., Mendrinos, S. & Taneja, S. S. Role of MRI in minimally invasive focal ablative therapy for prostate cancer. AJR Am. J. Roentgenol. 197, 90–96 (2011).
Haider, M. et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am. J. Roentgenol. 189, 323–328 (2007).
Lim, H., Kim, J., Kim, K. & Cho, K. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection—a multireader study. Radiology 250, 145–151 (2009).
Kim, C. K., Park, B. K., Lee, H. M. & Kwon, G. Y. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest. Radiol. 42, 842–847 (2007).
Vargas, H. A. et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259, 775–784 (2011).
Morgan, V., Kyriazi, S., Ashley, S. & DeSouza, N. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 48, 695–703 (2007).
Yoshimitsu, K. et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J. Magn. Reson. Imaging 27, 132–139 (2008).
Tanimoto, A., Nakashima, J., Kohno, H., Shinmoto, H. & Kuribayashi, S. Prostate cancer screening: The clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J. Magn. Reson. Imaging 25, 146–152 (2007).
Kitajima, K. et al. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J. Magn. Reson. Imaging 31, 625–631 (2010).
Delongchamps, N. B. et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 107, 1411–1418 (2011).
Mazaheri, Y. et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging--correlation with pathologic findings. Radiology 246, 480–488 (2008).
Jeong, I. G. et al. Diffusion-weighted magnetic resonance imaging in patients with unilateral prostate cancer on extended prostate biopsy: predictive accuracy of laterality and implications for hemi-ablative therapy. J. Urol. 184, 1963–1969 (2010).
Oto, A. et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 257, 715–723 (2010).
Ahmed, H. U. et al. Is it time to consider a role for MRI before prostate biopsy? Nat. Rev. Clin. Oncol. 6, 197–206 (2009).
Portalez, D. et al. Prospective comparison of T2w-MRI and dynamic-contrast-enhanced MRI, 3D-MR spectroscopic imaging or diffusion-weighted MRI in repeat TRUS-guided biopsies. Eur. Radiol. 20, 2781–2790 (2010).
Vilanova, J. C. et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-total prostate-specific antigen ratio in the detection of prostate cancer. AJR Am. J. Roentgenol. 196, W715–W722 (2011).
Hambrock, T. et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J. Urol. 183, 520–527 (2010).
Pinto, P. A. et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J. Urol. 186, 1281–1285 (2011).
Hadaschik, B. A. et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J. Urol. 186, 2214–2220 (2011).
Hambrock, T. et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur. Urol. 61, 177–184 (2012).
Augustin, H., Fritz, G., Ehammer, T., Auprich, M. & Pummer, K. Accuracy of 3-Tesla magnetic resonance imaging for the staging of prostate cancer in comparison to the Partin tables. Acta Radiol. 50, 562–569 (2009).
Hricak, H. et al. The role of preoperative endorectal magnetic resonance imaging in the decision regarding whether to preserve or resect neurovascular bundles during radical retropubic prostatectomy. Cancer 100, 2655–2663 (2004).
Ren, J. et al. Seminal vesicle invasion in prostate cancer: prediction with combined T2-weighted and diffusion-weighted MR imaging. Eur. Radiol. 19, 2481–2486 (2009).
Kim, C., Choi, D., Park, B., Kwon, G. & Lim, H. Diffusion-weighted MR imaging for the evaluation of seminal vesicle invasion in prostate cancer: initial results. J. Magn. Reson. Imaging 28, 963–969 (2008).
Ren, J. et al. Combined T2-weighted and diffusion-weighted MRI for diagnosis of urinary bladder invasion in patients with prostate carcinoma. J. Magn. Reson. Imaging 30, 351–356 (2009).
Luboldt, W. et al. Prostate carcinoma: diffusion-weighted imaging as potential alternative to conventional MR and 11C-choline PET/CT for detection of bone metastases. Radiology 249, 1017–1025 (2008).
Eiber, M. et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J. Magn. Reson. Imaging 33, 1160–1170 (2011).
Eiber, M. et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest. Radiol. 45, 15–23 (2010).
Gutzeit, A. et al. Comparison of diffusion-weighted whole body MRI and skeletal scintigraphy for the detection of bone metastases in patients with prostate or breast carcinoma. Skeletal Radiol. 39, 333–343 (2010).
Itou, Y., Nakanishi, K., Narumi, Y., Nishizawa, Y. & Tsukuma, H. Clinical utility of apparent diffusion coefficient (ADC) values in patients with prostate cancer: can ADC values contribute to assess the aggressiveness of prostate cancer? J. Magn. Reson. Imaging 33, 167–172 (2011).
Verma, S. et al. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am. J. Roentgenol. 196, 374–381 (2011).
Woodfield, C. et al. Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am. J. Roentgenol. 194, W316–W322 (2010).
Turkbey, B. et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 258, 488–495 (2011).
Langer, D. et al. Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2--sparse versus dense cancers. Radiology 249, 900–908 (2008).
Langer, D. L. et al. Prostate tissue composition and MR measurements: investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 255, 485–494 (2010).
D'Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
deSouza, N. et al. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumor aggressiveness in localized prostate cancer. Clin. Radiol. 63, 774–782 (2008).
Hambrock, T. et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259, 453–461 (2011).
van As, N. J. et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur. Urol. 56, 981–988 (2009).
Morgan, V. A. et al. Diffusion-weighted magnetic resonance imaging for monitoring prostate cancer progression in patients managed by active surveillance. Br. J. Radiol. 84, 31–37 (2011).
Giles, S. L. et al. Apparent diffusion coefficient as a predictive biomarker of prostate cancer progression: value of fast and slow diffusion components. AJR Am. J. Roentgenol. 196, 586–591 (2011).
Padhani, A. R. & Koh, D. M. Diffusion MR imaging for monitoring of treatment response. Magn. Res. Imaging Clin. N. Am. 19, 181–209 (2011).
Song, I., Kim, C., Park, B. & Park, W. Assessment of response to radiotherapy for prostate cancer: value of diffusion-weighted MRI at 3 T. AJR Am. J. Roentgenol. 194, W477–W482 (2010).
Wang, H. & Fei, B. Diffusion-weighted MRI for monitoring tumor response to photodynamic therapy. J. Magn. Reson. Imaging 32, 409–417 (2010).
Reischauer, C. et al. Bone metastases from prostate cancer: assessing treatment response by using diffusion-weighted imaging and functional diffusion maps--initial observations. Radiology 257, 523–531 (2010).
Kim, C., Park, B. & Lee, H. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. J. Magn. Reson. Imaging 29, 391–397 (2009).
Akin, O. et al. Incremental value of diffusion weighted and dynamic contrast enhanced MRI in the detection of locally recurrent prostate cancer after radiation treatment: preliminary results. Eur. Radiol. 21, 1970–1978 (2011).
Tamada, T. et al. Locally recurrent prostate cancer after high-dose-rate brachytherapy: the value of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging in localizing tumors. AJR Am. J. Roentgenol. 197, 408–414 (2011).
Nishida, K. et al. Incremental value of T2-weighted and diffusion-weighted MRI for prediction of biochemical recurrence after radical prostatectomy in clinically localized prostate cancer. Acta Radiol. 52, 120–126 (2011).
Park, S. Y., Kim, C. K., Park, B. K., Lee, H. M. & Lee, K. S. Prediction of biochemical recurrence following radical prostatectomy in men with prostate cancer by diffusion-weighted magnetic resonance imaging: initial results. Eur. Radiol. 21, 1111–1118 (2011).
Acknowledgements
The authors would like to thank the NIHR: Cambridge Biomedical Research Centre, the University of Cambridge, Hutchison Whampoa Limited, the Cambridge Experimental Cancer Medicine Centre, ACT, Cancer Research UK, and the Royal College of Surgeons of England for funding support.
Author information
Authors and Affiliations
Contributions
E. M. Lawrence and E. Sala researched the data and contributed towards writing the article, along with V. Gnanaprogasam. All authors contributed substantially to discussions of article content, as well as reviewing the editing the article prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lawrence, E., Gnanapragasam, V., Priest, A. et al. The emerging role of diffusion-weighted MRI in prostate cancer management. Nat Rev Urol 9, 94–101 (2012). https://doi.org/10.1038/nrurol.2011.222
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.222
This article is cited by
-
The Cambridge Prognostic Groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: a validation study
BMC Medicine (2018)
-
Targeted transperineal biopsy of the prostate has limited additional benefit over background cores for larger MRI-identified tumors
World Journal of Urology (2016)
-
Changing presentation of prostate cancer in a UK population – 10 year trends in prostate cancer risk profiles in the East of England
British Journal of Cancer (2013)