Abstract
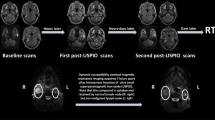
Nanotechnology is poised to have a substantial influence on biomedicine. A unique example of a nanotechnology that has progressed from proof-of-concept to human clinical trials is the use of ultrasmall superparamagnetic iron oxide nanoparticles as a cell-specific contrast agent for MRI. When injected systemically, these particles are taken up by macrophages of the reticuloendothelial system and accumulate in lymph nodes. This passive, cell-specific targeting of the iron oxide nanoparticles to lymph nodes, and the differential cellular content of benign versus malignantly infiltrated nodes make this method suitable for cancer staging. By using lymphotropic nanoparticle enhanced MRI, differences in benign versus malignant infiltration of lymph nodes can be visualized, which adds accuracy to standard MRI beyond criteria based solely upon the size and shape of lymph nodes. This technology has been used to assess lymph node metastases in a large number of human cancer types. In this Review, we focus on lymphotropic nanoparticle enhanced MRI and its application for the staging of genitourinary malignancies.
Key Points
-
Current imaging modalities, including CT, MRI, and PET have limited sensitivity and specificity for imaging lymph node metastases
-
Lymphotropic nanoparticle enhanced MRI (LNMRI) adds cell-specific information to conventional MRI
-
LNMRI might provide improved clinical assessment of lymph nodes in patients with genitourinary malignancies
-
Continued evaluation of LNMRI is necessary to broadly validate this technology in large multicenter trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mirkin, C. A., Letsinger, R. L., Mucic, R. C. & Storhoff, J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996).
Zhang, L. et al. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 83, 761–769 (2008).
Feldman, A. S., McDougal, W. S. & Harisinghani, M. G. The potential of nanoparticle-enhanced imaging. Urol. Oncol. 26, 65–73 (2008).
Gervasi, L. A. et al. Prognostic significance of lymph nodal metastases in prostate cancer. J. Urol. 142, 332–336 (1989).
Ries, L. A. G. Cancer survival among adults: US SEER program, 1988–2001, patient and tumor characteristics (US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda, MD, 2007).
Bader, P., Burkhard, F. C., Markwalder, R. & Studer, U. E. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J. Urol. 168, 514–518; discussion 518 (2002).
Sanderson, K. M., Skinner, D. & Stein, J. P. The prognostic and staging value of lymph node dissection in the treatment of invasive bladder cancer. Nat. Clin. Pract. Urol. 3, 485–494 (2006).
Guermazi, A., Brice, P., Hennequin, C. & Sarfati, E. Lymphography: an old technique retains its usefulness. Radiographics 23, 1541–1558; discussion 1559–1560 (2003).
Kramolowsky, E. V., Narayana, A. S., Platz, C. E. & Loening, S. A. The frozen section in lymphadenectomy for carcinoma of the prostate. J. Urol. 131, 899–900 (1984).
Catalona, W. J. & Stein, A. J. Accuracy of frozen section detection of lymph node metastases in prostatic carcinoma. J. Urol. 127, 460–461 (1982).
Campbell, S. C., Klein, E. A., Levin, H. S. & Piedmonte, M. R. Open pelvic lymph node dissection for prostate cancer: a reassessment. Urology 46, 352–355 (1995).
Tiguert, R. et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology 53, 367–371 (1999).
Yang, W. T., Lam, W. W., Yu, M. Y., Cheung, T. H. & Metreweli, C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am. J. Roentgenol. 175, 759–766 (2000).
Jager, G. J., Barentsz, J. O., Oosterhof, G. O., Witjes, J. A. & Ruijs, S. J. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am. J. Roentgenol. 167, 1503–1507 (1996).
Grubnic, S., Vinnicombe, S. J., Norman, A. R. & Husband, J. E. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin. Radiol. 57, 193–200; discussion 201–204 (2002).
Saksena, M. A., Saokar, A. & Harisinghani, M. G. Lymphotropic nanoparticle enhanced MR imaging (LNMRI) technique for lymph node imaging. Eur. J. Radiol. 58, 367–374 (2006).
Subak, L. L., Hricak, H., Powell, C. B., Azizi, L. & Stern, J. L. Cervical carcinoma: computed tomography and magnetic resonance imaging for preoperative staging. Obstet. Gynecol. 86, 43–50 (1995).
Borley, N. et al. Laparoscopic pelvic lymph node dissection allows significantly more accurate staging in “high-risk” prostate cancer compared to MRI or CT. Scand. J. Urol. Nephrol. 37, 382–386 (2003).
Hovels, A. M. et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol. 63, 387–395 (2008).
De Santis, M. et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J. Clin. Oncol. 22, 1034–1039 (2004).
De Santis, M. & Pont, J. The role of positron emission tomography in germ cell cancer. World J. Urol. 22, 41–46 (2004).
Wilson, C. B. et al. Imaging metastatic testicular germ cell tumours with 18FDG positron emission tomography: prospects for detection and management. Eur. J. Nucl. Med. 22, 508–513 (1995).
Albers, P. et al. Positron emission tomography in the clinical staging of patients with stage I and II testicular germ cell tumors. Urology 53, 808–811 (1999).
Salminen, E., Hogg, A., Binns, D., Frydenberg, M. & Hicks, R. Investigations with FDG-PET scanning in prostate cancer show limited value for clinical practice. Acta Oncol. 41, 425–429 (2002).
Sung, J., Espiritu, J. I., Segall, G. M. & Terris, M. K. Fluorodeoxyglucose positron emission tomography studies in the diagnosis and staging of clinically advanced prostate cancer. BJU Int. 92, 24–27 (2003).
Powles, T., Murray, I., Brock, C., Oliver, T. & Avril, N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur. Urol. 51, 1511–1520; discussion 1520–1521 (2007).
Schoder, H. & Larson, S. M. Positron emission tomography for prostate, bladder, and renal cancer. Semin. Nucl. Med. 34, 274–292 (2004).
Kurhanewicz, J. et al. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7 cm3) spatial resolution. Radiology 198, 795–805 (1996).
Kotzerke, J. et al. Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur. J. Nucl. Med. 27, 1415–1419 (2000).
Bockisch, A., Freudenberg, L. S., Schmidt, D. & Kuwert, T. Hybrid imaging by SPECT/CT and PET/CT: proven outcomes in cancer imaging. Semin. Nucl. Med. 39, 276–289 (2009).
Esen, G. Ultrasound of superficial lymph nodes. Eur. J. Radiol. 58, 345–359 (2006).
Crawshaw, J. W. et al. Sentinel lymph node biopsy using dynamic lymphoscintigraphy combined with ultrasound-guided fine needle aspiration in penile carcinoma. Br. J. Radiol. 82, 41–48 (2009).
Jung, C. W. & Jacobs, P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 13, 661–674 (1995).
Wang, Y. X., Hussain, S. M. & Krestin, G. P. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur. Radiol. 11, 2319–2331 (2001).
Harisinghani, M. G. et al. MR lymphangiography: imaging strategies to optimize the imaging of lymph nodes with ferumoxtran-10. Radiographics 24, 867–878 (2004).
Bernd, H., De Kerviler, E., Gaillard, S. & Bonnemain, B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest. Radiol. 44, 336–342 (2009).
Anzai, Y. et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology 228, 777–788 (2003).
Namasivayam, S., Kalra, M. K., Torres, W. E. & Small, W. C. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg. Radiol. 12, 210–215 (2006).
Dillman, J. R., Ellis, J. H., Cohan, R. H., Strouse, P. J. & Jan, S. C. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am. J. Roentgenol. 189, 1533–1538 (2007).
Murphy, K. P., Szopinski, K. T., Cohan, R. H., Mermillod, B. & Ellis, J. H. Occurrence of adverse reactions to gadolinium-based contrast material and management of patients at increased risk: a survey of the American Society of Neuroradiology Fellowship Directors. Acad. Radiol. 6, 656–664 (1999).
Morris, M. F. et al. Features of nephrogenic systemic fibrosis on radiology examinations. AJR Am. J. Roentgenol. 193, 61–69 (2009).
Thomsen, H. S. How to avoid nephrogenic systemic fibrosis: current guidelines in Europe and the United States. Radiol. Clin. North Am. 47, 871–875 (2009).
Guimaraes, A. R. et al. Pilot study evaluating use of lymphotrophic nanoparticle-enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology 71, 708–712 (2008).
Guimaraes, R., Clement, O., Bittoun, J., Carnot, F. & Frija, G. MR lymphography with superparamagnetic iron nanoparticles in rats: pathologic basis for contrast enhancement. AJR Am. J. Roentgenol. 162, 201–207 (1994).
Weissleder, R. et al. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175, 494–498 (1990).
Weissleder, R. et al. Ultrasmall superparamagnetic iron oxide: characterization of a new class of contrast agents for MR imaging. Radiology 175, 489–493 (1990).
Clement, O., Guimaraes, R., de Kerviler, E. & Frija, G. Magnetic resonance lymphography. Enhancement patterns using superparamagnetic nanoparticles. Invest. Radiol. 29 (Suppl. 2), S226–S228 (1994).
Harisinghani, M. G. & Weissleder, R. Sensitive, noninvasive detection of lymph node metastases. PLoS Med. 1, e66 (2004).
Harisinghani, M. G. et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 348, 2491–2499 (2003).
Pannu, H. K., Wang, K. P., Borman, T. L. & Bluemke, D. A. MR imaging of mediastinal lymph nodes: evaluation using a superparamagnetic contrast agent. J. Magn. Reson. Imaging 12, 899–904 (2000).
Bellin, M. F., Lebleu, L. & Meric, J. B. Evaluation of retroperitoneal and pelvic lymph node metastases with MRI and MR lymphangiography. Abdom. Imaging 28, 155–163 (2003).
Harisinghani, M. G. et al. MR lymphangiography for detection of minimal nodal disease in patients with prostate cancer. Acad. Radiol. 9 (Suppl. 2), S312–S313 (2002).
Cheng, L. et al. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 91, 66–73 (2001).
Smith, M. R. et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 345, 948–955 (2001).
Messing, E. M. et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N. Engl. J. Med. 341, 1781–1788 (1999).
Bolla, M. et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 337, 295–300 (1997).
Partin, A. W. et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J. Urol. 150, 110–114 (1993).
Kattan, M. W. Re: Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy gleason score (Partin Tables) based on cases from 2000 to 2005. Eur. Urol. 52, 1528 (2007).
Narayan, P. et al. Utility of preoperative serum prostate-specific antigen concentration and biopsy Gleason score in predicting risk of pelvic lymph node metastases in prostate cancer. Urology 44, 519–524 (1994).
Bishoff, J. T. et al. Pelvic lymphadenectomy can be omitted in selected patients with carcinoma of the prostate: development of a system of patient selection. Urology 45, 270–274 (1995).
Heesakkers, R. A. et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 9, 850–856 (2008).
Heesakkers, R. A. et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology 251, 408–414 (2009).
Ross, R. W. et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin. Imaging 33, 301–305 (2009).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Bader, P., Burkhard, F. C., Markwalder, R. & Studer, U. E. Disease progression and survival of patients with positive lymph nodes after radical prostatectomy. Is there a chance of cure? J. Urol. 169, 849–854 (2003).
Fleischmann, A., Thalmann, G. N., Markwalder, R. & Studer, U. E. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J. Clin. Oncol. 23, 2358–2365 (2005).
Schumacher, M. C., Burkhard, F. C., Thalmann, G. N., Fleischmann, A. & Studer, U. E. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur. Urol. 54, 344–352 (2008).
Mills, R. D., Fleischmann, A. & Studer, U. E. Radical cystectomy with an extended pelvic lymphadenectomy: rationale and results. Surg. Oncol. Clin. N. Am. 16, 233–245 (2007).
Deserno, W. M. et al. Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10-enhanced MR imaging. Radiology 233, 449–456 (2004).
Thoeny, H. C. et al. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur. Urol. 55, 761–769 (2009).
McDougal, W. S. Carcinoma of the penis: improved survival by early regional lymphadenectomy based on the histological grade and depth of invasion of the primary lesion. J. Urol. 154, 1364–1366 (1995).
Ornellas, A. A. et al. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J. Urol. 151, 1244–1249 (1994).
Tabatabaei, S., Harisinghani, M. & McDougal, W. S. Regional lymph node staging using lymphotropic nanoparticle enhanced magnetic resonance imaging with ferumoxtran-10 in patients with penile cancer. J. Urol. 174, 923–927; discussion 927 (2005).
Jemal, A. et al. Cancer statistics, 2005. CA Cancer J. Clin. 55, 10–30 (2005).
Jemal, A. et al. Cancer statistics, 2004. CA Cancer J. Clin. 54, 8–29 (2004).
Richie, J. P., Garnick, M. B. & Finberg, H. Computerized tomography: how accurate for abdominal staging of testis tumors? J. Urol. 127, 715–717 (1982).
Harisinghani, M. G. et al. A pilot study of lymphotrophic nanoparticle-enhanced magnetic resonance imaging technique in early stage testicular cancer: a new method for noninvasive lymph node evaluation. Urology 66, 1066–1071 (2005).
Pantuck, A. J., Zisman, A. & Belldegrun, A. S. The changing natural history of renal cell carcinoma. J. Urol. 166, 1611–1623 (2001).
Pantuck, A. J. et al. Renal cell carcinoma with retroperitoneal lymph nodes. Impact on survival and benefits of immunotherapy. Cancer 97, 2995–3002 (2003).
Robson, C. J., Churchill, B. M. & Anderson, W. The results of radical nephrectomy for renal cell carcinoma. J. Urol. 101, 297–301 (1969).
DeKernion, J. B. Lymphadenectomy for renal cell carcinoma. Therapeutic implications. Urol. Clin. North Am. 7, 697–703 (1980).
Freedland, S. J. & Dekernion, J. B. Role of lymphadenectomy for patients undergoing radical nephrectomy for renal cell carcinoma. Rev. Urol. 5, 191–195 (2003).
Giberti, C., Oneto, F., Martorana, G., Rovida, S. & Carmignani, G. Radical nephrectomy for renal cell carcinoma: long-term results and prognostic factors on a series of 328 cases. Eur. Urol. 31, 40–48 (1997).
Giuliani, L., Martorana, G., Giberti, C., Pescatore, D. & Magnani, G. Results of radical nephrectomy with extensive lymphadenectomy for renal cell carcinoma. J. Urol. 130, 664–668 (1983).
Ferrigni, R. G. & Novicki, D. E. Chylous ascites complicating genitourinary oncological surgery. J. Urol. 134, 774–776 (1985).
Herrlinger, A., Schrott, K. M., Schott, G. & Sigel, A. What are the benefits of extended dissection of the regional renal lymph nodes in the therapy of renal cell carcinoma. J. Urol. 146, 1224–1227 (1991).
Pantuck, A. J. et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J. Urol. 169, 2076–2083 (2003).
Schafhauser, W., Ebert, A., Brod, J., Petsch, S. & Schrott, K. M. Lymph node involvement in renal cell carcinoma and survival chance by systematic lymphadenectomy. Anticancer Res. 19, 1573–1578 (1999).
Harisinghani, M. G. et al. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization? AJR Am. J. Roentgenol. 186, 144–148 (2006).
Will, O. et al. Diagnostic precision of nanoparticle-enhanced MRI for lymph-node metastases: a meta-analysis. Lancet Oncol. 7, 52–60 (2006).
Pandharipande, P. V. et al. Lymphotropic nanoparticle-enhanced MRI for independent prediction of lymph node malignancy: a logistic regression model. AJR Am. J. Roentgenol. 193, W230–W237 (2009).
Harisinghani, M., Ross, R. W., Guimaraes, A. R. & Weissleder, R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia 9, 1160–1165 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C. S. Thaxton declares that he is a co-founder of AuraSense, a start-up biotechnology company focusing on the commercialization of therapeutic and theranostic nanoparticles, and a shareholder in Nanosphere, a nanotechnology biodiagnostic company. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mouli, S., Zhao, L., Omary, R. et al. Lymphotropic nanoparticle enhanced MRI for the staging of genitourinary tumors. Nat Rev Urol 7, 84–93 (2010). https://doi.org/10.1038/nrurol.2009.254
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2009.254
This article is cited by
-
Towards clinically translatable in vivo nanodiagnostics
Nature Reviews Materials (2017)
-
Imaging macrophages with nanoparticles
Nature Materials (2014)
-
Preclinical Lymphatic Imaging
Molecular Imaging and Biology (2011)