Key Points
-
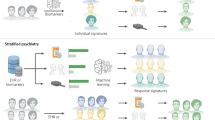
Stratified medicine focuses on the scientifically based classification of disease and treatment response, and is well suited to the practice of neurology
-
Stratification of patients on the basis of genetic and clinical characteristics and imaging has already had an impact on the cost-effectiveness of preventative and therapeutic interventions in patients with primary and secondary stroke
-
Pharmacogenetic stratification has been introduced to manage warfarin treatment in patients at risk of stroke and to reduce risks of severe cutaneous reactions in patients treated with antiepileptic drugs
-
Stratification of patients with multiple sclerosis on the basis of baseline characteristics and short-term pharmacodynamic responses could increase treatment benefits, limit adverse effects and reduce the costs of treatment
-
Brain imaging and other biomarkers enable specific diagnoses of dementia syndromes, and could be used to stratify healthy individuals at risk of developing dementia for early preventative interventions
-
Major challenges to clinical implementation of stratified neurology exist, but its high value, together with technological and scientific advances, justify a concerted effort to accelerate development in this area
Abstract
Stratified medicine can reduce the costs of neurological care, bringing benefits to both patients and physicians. The availability of routine genetic testing, new biomarkers and advanced imaging, as well as new technologies for patient-centred data collection, has expanded the potential for patient stratification. Several neurology subspecialities, including stroke, epilepsy and behavioural neurology, have already applied stratification for disease prognosis, optimization of disease management and reduction of treatment-related adverse events. Stratification approaches could improve the cost-effectiveness of neurological care that involves treatments with high costs or risks of adverse reactions, as well as guide the use of emerging, highly individualized therapies. There are still major challenges in the development of clinically actionable stratification concepts, and practical barriers can limit adoption of these concepts into clinical practice. However, improved technologies and disease understanding are making more precise stratification practical. We believe that neurologists should become leaders in the development and validation of these practices, and that use of these approaches should be part of a broader strategy for addressing both the growing needs of an ageing population and the rising pressures for rapid improvements in the cost-effectiveness of therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Trusheim, M. R., Berndt, E. R. & Douglas, F. L. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat. Rev. Drug Discov. 6, 287–293 (2007).
Horwitz, R. I., Cullen, M. R., Abell, J. & Christian, J. B. (De)personalized medicine. Science 339, 1155–1156 (2013).
Schilsky, R. L. Personalized medicine in oncology: the future is now. Nat. Rev. Drug Discov. 9, 363–366 (2010).
Saver, J. L. et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke 40, 2433–2437 (2009).
Costa, J. et al. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia 52, 1280–1291 (2011).
Romero, J. R. Prevention of ischemic stroke: overview of traditional risk factors. Curr. Drug Targets 8, 794–801 (2007).
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis (North American Symptomatic Carotid Endarterectomy Trial Collaborators). N. Engl. J. Med. 325, 445–453 (1991).
[No authors listed] Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 273, 1421–1428 (1995).
Sitzer, M. et al. Plaque ulceration and lumen thrombus are the main sources of cerebral microemboli in high-grade internal carotid artery stenosis. Stroke 26, 1231–1233 (1995).
Johnston, S. C. et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369, 283–292 (2007).
Gage, B. F. et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285, 2864–2870 (2001).
Lip, G. Y., Nieuwlaat, R., Pisters, R., Lane, D. A. & Crijns, H. J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 137, 263–272 (2010).
Pisters, R. et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 138, 1093–1100 (2010).
Carlquist, J. F. & Anderson, J. L. Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation 124, 2554–2559 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Lou, M. et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology 71, 1417–1423 (2008).
Cucchiara, B., Tanne, D., Levine, S. R., Demchuk, A. M. & Kasner, S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 17, 331–333 (2008).
Strbian, D. et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology 78, 427–432 (2012).
Stroke Outcomes Research Canada. Ischemic Stroke Predictive Risk Score [online], (2012).
Chung, W. H. & Hung, S. I. Recent advances in the genetics and immunology of Stevens–Johnson syndrome and toxic epidermal necrosis. J. Dermatol. Sci. 66, 190–196 (2012).
Ferrell, P. B. Jr & McLeod, H. L. Carbamazepine, HLA-B*1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 9, 1543–1546 (2008).
Yip, V. L., Marson, A. G., Jorgensen, A. L., Pirmohamed, M. & Alfirevic, A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clin. Pharmacol. Ther. 92, 757–765 (2012).
Hung, S. I. et al. Common risk allele in aromatic antiepileptic-drug induced Stevens–Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 11, 349–356 (2010).
US Department of Health and Human Services. Information for healthcare professionals: dangerous or even fatal skin reactions—carbamazepine (marketed as Carbatrol, Equetro, Tegretol, and generics). US Food and Drug Administration [online], (2007).
Medicines and Healthcare Products Regulators Agency. Carbamazepine: genetic testing recommended in some Asian populations. Medicines and Healthcare Products Regulators Agency [online], (2008).
Dong, D., Sung, C. & Finkelstein, E. A. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 79, 1259–1267 (2012).
Hung, S. I. & Chung, W. H. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 80, 1621 (2013).
Ozeki, T. et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 20, 1034–1041 (2011).
McCormack, M. et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 364, 1134–1143 (2011).
Franciotta, D., Kwan, P. & Perucca, E. Genetic basis for idiosyncratic reactions to antiepileptic drugs. Curr. Opin. Neurol. 22, 144–149 (2009).
Johnson, M. R., Tan, N. C., Kwan, P. & Brodie, M. J. Newly diagnosed epilepsy and pharmacogenomics research: a step in the right direction? Epilepsy Behav. 22, 3–8 (2011).
Chan, A., Pirmohamed, M. & Comabella, M. Pharmacogenomics in neurology: current state and future steps. Ann. Neurol. 70, 684–697 (2011).
Kwan, P. & Brodie, M. J. Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319 (2000).
Johnson, M. R. et al. A twin study of genetic influences on epilepsy outcome. Twin Res. 6, 140–146 (2003).
Speed, D. A genome-wide association study and biological pathway analysis of epilepsy prognosis in a prospective cohort of newly treated epilepsy. Hum. Mol. Genet. http://dx.doi.org/10.1093/hmg/ddt403.
De Jonghe, P. Molecular genetics of Dravet syndrome. Dev. Med. Child Neurol. 53 (Suppl. 2), 7–10 (2011).
Epi, K. C. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
Weinshenker, B. G. et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 112, 133–146 (1989).
Derfuss, T. Personalized medicine in multiple sclerosis: hope or reality? BMC Med. 10, 116 (2012).
Miller, A., Avidan, N., Tzunz-Henig, N. & Glass-Marmor, L. Translation towards personalized medicine in multiple sclerosis. J. Neurol. Sci. 274, 68–75 (2008).
Sormani, M. P. & De Stefano, N. Defining and scoring response to IFN-β. Nat. Rev. Neurol. 9, 504–512 (2013).
Rio, J., Comabella, M. & Montalban, X. Predicting responders to therapies for multiple sclerosis. Nat. Rev. Neurol. 5, 553–560 (2009).
Koch, M. W., Metz, L. M., Agrawal, S. M. & Yong, V. W. Environmental factors and their regulation of immunity in multiple sclerosis. J. Neurol. Sci. 324, 10–16 (2013).
Ramagopalan, S. V. et al. Association of smoking with risk of multiple sclerosis: a population-based study. J. Neurol. 260, 1778–1781 (2013).
Montalban, X. et al. MRI criteria for MS in patients with clinically isolated syndromes. Neurology 74, 427–434 (2010).
Sormani, M. P., Rovaris, M., Comi, G. & Filippi, M. A reassessment of the plateauing relationship between T2 lesion load and disability in MS. Neurology 73, 1538–1542 (2009).
Bielekova, B. et al. MRI as a marker for disease heterogeneity in multiple sclerosis. Neurology 65, 1071–1076 (2005).
Rovaris, M. et al. Relationship between brain MRI lesion load and short-term disease evolution in non-disabling MS: a large-scale, multicentre study. Mult. Scler. 17, 319–326 (2011).
Fisniku, L. K. et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 131, 808–817 (2008).
Sormani, M. P. et al. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann. Neurol. 65, 268–275 (2009).
Sormani, M. P., Arnold, D. L. & De Stefano, N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann. Neurol. http://dx.doi.org/10.1002/ana.24018.
Owen, D. R. & Matthews, P. M. Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands. Int. Rev. Neurobiol. 101, 19–39 (2011).
Politis, M. et al. Increased PK11195 PET binding in the cortex of patients with MS correlates with disability. Neurology 79, 523–530 (2012).
Farrell, R. A. et al. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 73, 32–38 (2009).
Lunemann, J. D. et al. Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 67, 159–169 (2010).
Wandinger, K. et al. Association between clinical disease activity and Epstein–Barr virus reactivation in MS. Neurology 55, 178–184 (2000).
Ascherio, A. & Marrie, R. A. Vitamin D in MS: a vitamin for 4 seasons. Neurology 79, 208–210 (2012).
Kebir, H. et al. Preferential recruitment of interferon-γ-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 66, 390–402 (2009).
Bushnell, S. E. et al. Serum IL-17F does not predict poor response to IM IFNβ-1a in relapsing-remitting MS. Neurology 79, 531–537 (2012).
Avolio, C. et al. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-β-1a treatment. Mult. Scler. 11, 441–446 (2005).
Villar, L. M. et al. Intrathecal IgM synthesis is a prognostic factor in multiple sclerosis. Ann. Neurol. 53, 222–226 (2003).
Khademi, M. et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult. Scler. 17, 335–343 (2011).
Comabella, M. et al. Plasma osteopontin levels in multiple sclerosis. J. Neuroimmunol. 158, 231–239 (2005).
Axelsson, M. et al. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. J. Neurol. 258, 882–888 (2011).
Gunnarsson, M. et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 69, 83–89 (2011).
Norgren, N. et al. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 63, 1586–1590 (2004).
Grossman, I. et al. Pharmacogenetics of glatiramer acetate therapy for multiple sclerosis reveals drug-response markers. Pharmacogenet. Genomics 17, 657–666 (2007).
Hesse, D., Sellebjerg, F. & Sorensen, P. S. Absence of MxA induction by interferon beta in patients with MS reflects complete loss of bioactivity. Neurology 73, 372–377 (2009).
Fainardi, E. et al. Presence of detectable levels of soluble HLA-G molecules in CSF of relapsing-remitting multiple sclerosis: relationship with CSF soluble HLA-I and IL-10 concentrations and MRI findings. J. Neuroimmunol. 142, 149–158 (2003).
Polman, C. H. et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 9, 740–750 (2010).
Goodin, D. S. et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 68, 977–984 (2007).
Baranzini, S. E. et al. Transcription-based prediction of response to IFNβ using supervised computational methods. PLoS Biol. 3, e2 (2005).
Baarsen, L. G. & Baarsen, V. Pharmacogenomics of IFN-β in multiple sclerosis: towards a personalized medicine approach. Pharmacogenomics 10, 97–108 (2009).
Sturzebecher, S. et al. Expression profiling identifies responder and non-responder phenotypes to interferon-beta in multiple sclerosis. Brain 126, 1419–1429 (2003).
Cribbs, D. H. et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation 9, 179 (2012).
Jones, J. L. et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J. Clin. Invest. 119, 2052–2061 (2009).
Feldman, H. H. et al. Diagnosis and treatment of dementia: 2. Diagnosis. CMAJ 178, 825–836 (2008).
Jack, C. R. Jr et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 257–262 (2011).
Kantarci, K. et al. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology 77, 951–958 (2011).
Nordberg, A., Rinne, J. O., Kadir, A. & Langstrom, B. The use of PET in Alzheimer disease. Nat. Rev. Neurol. 6, 78–87 (2010).
Weiner, M. W. et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 8, S1–S68 (2012).
Pike, K. E. et al. Cognition and beta-amyloid in preclinical Alzheimer's disease: data from the AIBL study. Neuropsychologia 49, 2384–2390 (2011).
Nussbaum, R. L. Genome-wide association studies, Alzheimer disease, and understudied populations. JAMA 309, 1527–1528 (2013).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat. Genet. http://dx.doi.org/10.1038/ng.2802.
Goate, A. & Hardy, J. Twenty years of Alzheimer's disease-causing mutations. J. Neurochem. 120 (Suppl. 1), 3–8 (2012).
Sanan, D. A. et al. Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J. Clin. Invest. 94, 860–869 (1994).
Weisgraber, K. H. & Mahley, R. W. Human apolipoprotein E: the Alzheimer's disease connection. FASEB J. 10, 1485–1494 (1996).
Toledo, J. B. et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 136, 2697–2706 (2013).
Harold, D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41, 1088–1093 (2009).
Hollingworth, P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 43, 429–435 (2011).
Critical Path Institute. Alzheimer's Disease Clinical Trial Simulation Tool. Critical Path Institute [online].
Klunk, W. E. et al. The binding of 2-(4′-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J. Neurosci. 23, 2086–2092 (2003).
Klunk, W. E. Amyloid imaging as a biomarker for cerebral beta-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol. Aging 32 (Suppl. 1), S20–S36 (2011).
Edison, P. et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68, 501–508 (2007).
Pike, K. E. et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 130, 2837–2844 (2007).
Jack, C. R. Jr et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Fagan, A. M. et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 59, 512–519 (2006).
Buerger, K. et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain 129, 3035–3041 (2006).
Hampel, H. et al. Biomarkers for Alzheimer's disease therapeutic trials. Prog. Neurobiol. 95, 579–593 (2011).
Whitwell, J. L. et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case-control study. Lancet Neurol. 11, 868–877 (2012).
Ryan, N. S. & Fox, N. C. Imaging biomarkers in Alzheimer's disease. Ann. N. Y. Acad. Sci. 1180, 20–27 (2009).
Douaud, G. et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J. Neurosci. 33, 2147–2155 (2013).
Landau, S. M. et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 32, 1207–1218 (2011).
Landau, S. M. et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–238 (2010).
Sabate, E. Adherence to long-term therapies. Evidence for action (World Health Organisation, 2003).
Hamburg, M. A. & Collins, F. S. The path to personalized medicine. N. Engl. J. Med. 363, 301–304 (2010).
Hobart, J. C., Cano, S. J., Zajicek, J. P. & Thompson, A. J. Rating scales as outcome measures for clinical trials in neurology: problems, solutions, and recommendations. Lancet Neurol. 6, 1094–1105 (2007).
Bristol-Myers Squibb Company. Full prescribing information: Coumadin (warfarin sodium) (Bristol-Myers Squibb, 2011).
Marburger, J. H. & Kvamme, E. F. Report of the President's Council of Advisors on Science and Technology (ed. US President's Council of Advisors on Science and Technology (PCOST); Washington, 2008).
Committee on a Framework for Development of a New Taxonomy of Disease; National Research Council. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease (The National Academies Press, 2013).
Hood, L. & Friend, S. H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 8, 184–187 (2011).
Puetz, V., Dzialowski, I., Hill, M. D. & Demchuk, A. M. The Alberta Stroke Program Early CT Score in clinical practice: what have we learned? Stroke 4, 354–364 (2009).
Bermel, R. A. et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann. Neurol. 73, 95–103 (2013).
Wei, X. et al. Targeted next-generation sequencing as a comprehensive test for patients with and female carriers of DMD/BMD: a multi-population diagnostic study. Eur. J. Hum. Genet. http://dx.doi.org/10.1038/ejhg.2013.82.
Vasli, N. et al. Next generation sequencing for molecular diagnosis of neuromuscular diseases. Acta Neuropathol. 124, 273–283 (2012).
Vasli, N. & Laporte, J. Impacts of massively parallel sequencing for genetic diagnosis of neuromuscular disorders. Acta Neuropathol. 125, 173–185 (2013).
Dziubianau, M. et al. TCR repertoire analysis by next generation sequencing allows complex differential diagnosis of T cell-related pathology. Am. J. Transplant. http://dx.doi.org/10.1111/ajt.12431.
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Marrie, R. A. et al. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 72, 117–124 (2009).
Marrie, R. A. et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 74, 1041–1047 (2010).
Banerjee, A. K. & Ingate, S. Web-based patient-reported outcomes in drug safety and risk management: challenges and opportunities? Drug Saf. 35, 437–446 (2012).
Willke, R. J., Burke, L. B. & Erickson, P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control. Clin. Trials 25, 535–552 (2004).
van Ommen, G. J., van Deutekom, J. & Aartsma-Rus, A. The therapeutic potential of antisense-mediated exon skipping. Curr. Opin. Mol. Ther. 10, 140–149 (2008).
Nakamura, A. & Takeda, S. Exon-skipping therapy for Duchenne muscular dystrophy. Lancet 378, 546–547 (2011).
Kanagawa, M. & Toda, T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J. Hum. Genet. 51, 915–926 (2006).
Mitrpant, C., Fletcher, S. & Wilton, S. D. Personalised genetic intervention for Duchenne muscular dystrophy: antisense oligomers and exon skipping. Curr. Mol. Pharmacol. 2, 110–121 (2009).
Cox, T. M. & Cachon-Gonzalez, M. B. The cellular pathology of lysosomal diseases. J. Pathol. 226, 241–254 (2012).
Suzuki, Y. Chaperone therapy update: Fabry disease, GM1-gangliosidosis and Gaucher disease. Brain Dev. 35, 515–523 (2013).
Germain, D. P. et al. Safety and pharmacodynamic effects of a pharmacological chaperone on alpha-galactosidase A activity and globotriaosylceramide clearance in Fabry disease: report from two phase 2 clinical studies. Orphanet J. Rare Dis. 7, 91 (2012).
Davis, J. C. et al. The microeconomics of personalized medicine: today's challenge and tomorrow's promise. Nat. Rev. Drug Discov. 8, 279–286 (2009).
Acknowledgements
P. M. Matthews is grateful for support from the Medical Research Council to develop the OPTIMISE consortium for stratified medicine in MS. He received considerable input concerning stratified medicine approaches from many involved in the consortium, particularly R. Horwitz and J. Abell (GSK), R. Hyde (Biogen), G. Giovanonni (Queen Mary College London), L. Fugger and M. Craner (University of Oxford), C. Green (Exeter University), M. P. Sormani (Genoa University), D. Miller (University College London) and S. Baranzini (UCSF). This work also benefited from support to Imperial College London from the UK NIHR Biomedical Research Centres Scheme.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and made substantial contributions to discussion of the content, writing of the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P. M. Matthews is a part-time employee of GlaxoSmithKline Research and Development, Ltd, and holds stocks and options in the company. He has received speakers honoraria from Novartis and Biogen. P. Edison has received consultancy honoraria from Piramal Life Sciences. M. R. Johnson has received Advisory Board and speakers honoraria from GlaxoSmithKline, UCB Pharma and Esai.
Rights and permissions
About this article
Cite this article
Matthews, P., Edison, P., Geraghty, O. et al. The emerging agenda of stratified medicine in neurology. Nat Rev Neurol 10, 15–26 (2014). https://doi.org/10.1038/nrneurol.2013.245
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2013.245
This article is cited by
-
Nitric oxide and the brain. Part 1: Mechanisms of regulation, transport and effects on the developing brain
Pediatric Research (2021)
-
Practices and views of neurologists regarding the use of whole-genome sequencing in clinical settings: a web-based survey
European Journal of Human Genetics (2017)
-
Transforming Healthcare Delivery: Integrating Dynamic Simulation Modelling and Big Data in Health Economics and Outcomes Research
PharmacoEconomics (2016)
-
Service users’ and carers’ views on research towards stratified medicine in psychiatry: a qualitative study
BMC Research Notes (2015)