Key Points
-
'Type III secretion' (TTS) is a mechanism by which Gram-negative bacteria that are either extracellular or localized in a phagosome communicate with eukaryotic cells by injecting bacterial proteins across cellular membranes into the cytosol of these cells.
-
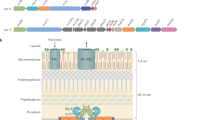
The Ysc injectisome of Yersinia is an organelle that spans the peptidoglycan layer and the two bacterial membranes, and is topped by a needle-like structure that protrudes outside the bacterium. The plasmid-encoded Ysc–Yop TTS system allows extracellular Yersinia docked at the surface of cells of the immune system to deliver Yop 'effectors' into the cytosol of these cells. Yops are recognized specifically by the Ysc injectisome by a signal that is located in the first few residues/codons of the protein/gene.
-
Several Yops need the assistance of specialized chaperones to be secreted by the injectisome, and secretion is triggered by intimate contact with a eukaryotic target cell. Translocation of the effector Yops across the eukaryotic cell membrane requires other Yops (YopB, YopD and LcrV), known as 'translocator' Yops, and these translocators form pores in the membrane of the target cells.
-
The effector Yops YopH, YopE, YopT and YpkA/YopO paralyse phagocytes. YopH is a tyrosine phosphatase that dephosphorylates focal adhesion complexes and a complex that comprises Fyb and SKAP-HOM. YopE is a GTPase-activating protein (GAP) for the Rho family of GTPases. YopT is a protease that cleaves the carboxyl terminus of members of the same Rho GTPase family, which detaches them from their prenyl membrane anchor. YopO/YpkA is a serine/threonine kinase that is activated by actin.
-
The effector Yops YopP and YopH block the pro-inflammatory response of infected cells. YopP is a protease, possibly a SUMO-protease, that blocks the nuclear factor-κB and mitogen-activated protein kinase pathways and hence the release of pro-inflammatory cytokines and adhesion molecules. YopH blocks the activation of the phosphatidylinositol 3-kinase pathway, which prevents lymphocyte proliferation and macrophage recruitment.
-
YopM is a protein with leucine-rich repeats that migrates to the nucleus. Although it is an important virulence factor, its target(s) and role(s) are unknown.
-
LcrV is an important Yop protein that has many functions — for example, as a regulator, a translocator and an extracellular anti-inflammatory agent.
Abstract
'Type III secretion' — the mechanism by which some pathogenic bacteria inject proteins straight into the cytosol of eukaryotic cells to 'anaesthetize' or 'enslave' them — was discovered in 1994. Important progress has been made in this area during the past few years: the bacterial organelles responsible for this secretion — called 'injectisomes' — have been visualized, the structures of some of the bacterial protein 'effectors' have been determined, and considerable progress has been made in understanding the intracellular action of the effectors. Type III secretion is key to the pathogenesis of bacteria from the Yersinia genus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rosqvist, R., Magnusson, K. E. & Wolf-Watz, H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13, 964–972 (1994).
Sory, M. P. & Cornelis, G. R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14, 583–594 (1994).A demonstration of the 'type III' concept of 'secretion–translocation' by the adenylate cyclase reporter strategy.
Cornelis, G. R. & Van Gijsegem, F. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54, 735–774 (2000).
Galan, J. E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322–1328 (1999).
Buttner, D. & Bonas, U. Port of entry — the type III secretion translocon. Trends Microbiol. 10, 186–192 (2002).
Hueck, C. J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433 (1998).
Cornelis, G. R. & Wolf-Watz, H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23, 861–867 (1997).
Bliska, J. B. & Black, D. S. Inhibition of the Fc receptor-mediated oxidative burst in macrophages by the Yersinia pseudotuberculosis tyrosine phosphatase. Infect. Immun. 63, 681–685 (1995).
Fallman, M. et al. Yersinia pseudotuberculosis inhibits Fc receptor-mediated phagocytosis in J774 cells. Infect. Immun. 63, 3117–3124 (1995).
Persson, C., Carballeira, N., Wolf-Watz, H. & Fallman, M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16, 2307–2318 (1997).
Rosqvist, R., Forsberg, A., Rimpilainen, M., Bergman, T. & Wolf-Watz, H. The cytotoxic protein YopE of Yersinia obstructs the primary host defence. Mol. Microbiol. 4, 657–667 (1990).
Visser, L. G., Annema, A. & van Furth, R. Role of Yops in inhibition of phagocytosis and killing of opsonized Yersinia enterocolitica by human granulocytes. Infect. Immun. 63, 2570–2575 (1995).
Schulte, R., Wattiau, P., Hartland, E. L., Robins-Browne, R. M. & Cornelis, G. R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64, 2106–2113 (1996).
Palmer, L. E., Hobbie, S., Galan, J. E. & Bliska, J. B. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNF-α production and downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27, 953–965 (1998).
Boland, A. & Cornelis, G. R. Role of YopP in suppression of tumor necrosis factor α release by macrophages during Yersinia infection. Infect. Immun. 66, 1878–1884 (1998).
Denecker, G. et al. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 70, 3510–3520 (2002).
Simonet, M., Richard, S. & Berche, P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect. Immun. 58, 841–845 (1990).
Cornelis, G. R. et al. The Yersinia yop regulon. Mol. Microbiol. 3, 1455–1459 (1989).
Rosqvist, R., Forsberg, A. & Wolf-Watz, H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59, 4562–4569 (1991).First description of the 'type III secretion–translocation' concept.
Hakansson, S. et al. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15, 5812–5823 (1996).
Neyt, C. & Cornelis, G. R. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33, 971–981 (1999).
Iriarte, M. & Cornelis, G. R. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29, 915–929 (1998).
Hakansson, S., Galyov, E. E., Rosqvist, R. & Wolf-Watz, H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20, 593–603 (1996).
Andor, A. et al. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol. 3, 301–310 (2001).
Barz, C., Abahji, T. N., Trulzsch, K. & Heesemann, J. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482, 139–143 (2000).
Black, D. S. & Bliska, J. B. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37, 515–527 (2000).
Zumbihl, R. et al. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274, 29289–29293 (1999).
Shao, F., Merritt, P. M., Bao, Z., Innes, R. W. & Dixon, J. E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109, 575–588 (2002).
Guan, K. L. & Dixon, J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science 249, 553–556 (1990).First identification of the enzymatic activity of an effector Yop and, more broadly, of a type III effector.
Black, D. S. & Bliska, J. B. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16, 2730–2744 (1997).
Black, D. S., Marie-Cardine, A., Schraven, B. & Bliska, J. B. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2, 401–414 (2000).
Yao, T., Mecsas, J., Healy, J. I., Falkow, S. & Chien, Y. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 190, 1343–1350 (1999).
Sauvonnet, N., Lambermont, I., Van der Bruggen, P. & Cornelis, G. R. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T-cell proliferation through inactivation of the phosphatidylinositol 3-kinase pathway. Mol. Microbiol. 45, 805–815 (2002).
Ruckdeschel, K. et al. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-α production. J. Biol. Chem. 272, 15920–15927 (1997).
Schesser, K. et al. The yopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28, 1067–1079 (1998).
Skrzypek, E., Cowan, C. & Straley, S. C. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30, 1051–1065 (1998).
Michiels, T. et al. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173, 4994–5009 (1991).
Salmond, G. P. & Reeves, P. J. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem. Sci. 18, 7–12 (1993).
Kubori, T. et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605 (1998).A visualization of the Salmonella injectisome.
Blocker, A. et al. Structure and composition of the Shigella flexneri 'needle complex', a part of its type III secreton. Mol. Microbiol. 39, 652–663 (2001).
Kimbrough, T. G. & Miller, S. I. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl Acad. Sci. USA 97, 11008–11013 (2000).
Kubori, T., Sukhan, A., Aizawa, S. I. & Galan, J. E. Molecular characterization and assembly of the needle complex of the Salmonella typhimurium type III protein secretion system. Proc. Natl Acad. Sci. USA 97, 10225–10230 (2000).
Hoiczyk, E. & Blobel, G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl Acad. Sci. USA 98, 4669–4674 (2001).
Woestyn, S., Allaoui, A., Wattiau, P. & Cornelis, G. R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J. Bacteriol. 176, 1561–1569 (1994).
Koster, M. et al. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26, 789–797 (1997).
Allaoui, A., Schulte, R. & Cornelis, G. R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol. Microbiol. 18, 343–355 (1995).
Cornelis, G., Vanootegem, J. C. & Sluiters, C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb. Pathog. 2, 367–379 (1987).Localization of the genes encoding YopE, YopH, YopD, YopO and YopP, the study of their expression and the identification of the master regulatory gene virF . This paper discusses how export might control the synthesis of the Yops.
Pettersson, J. et al. Modulation of virulence factor expression by pathogen target cell contact. Science 273, 1231–1233 (1996).Demonstration of the contact-inducibility of 'type III secretion–translocation'.
Boyd, A. P. et al. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79, 659–671 (2000).
Forsberg, A., Viitanen, A. M., Skurnik, M. & Wolf-Watz, H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5, 977–986 (1991).
Skryzpek, E. & Straley, S. C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175, 3520–3528 (1993).
Viboud, G. I. & Bliska, J. B. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20, 5373–5382 (2001).
Tardy, F. et al. Yersinia enterocolitica type III secretion–translocation system: channel formation by secreted Yops. EMBO J. 18, 6793–6799 (1999).
Sarker, M. R., Sory, M. P., Boyd, A. P., Iriarte, M. & Cornelis, G. R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect. Immun. 66, 2976–2979 (1998).
Pettersson, J. et al. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32, 961–976 (1999).
Fields, K. A., Nilles, M. L., Cowan, C. & Straley, S. C. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67, 5395–5408 (1999).
Lee, V. T., Tam, C. & Schneewind, O. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275, 36869–36875 (2000).
Holmstrom, A. et al. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39, 620–632 (2001).
Nilles, M. L., Fields, K. A. & Straley, S. C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180, 3410–3420 (1998).
Francis, M. S., Lloyd, S. A. & Wolf-Watz, H. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42, 1075–1093 (2001).
Boland, A. et al. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1. 8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 15, 5191–5201 (1996).
Boyd, A. P., Lambermont, I. & Cornelis, G. R. Competition between the Yops of Yersinia enterocolitica for delivery into eukaryotic cells: role of the SycE chaperone binding domain of YopE. J. Bacteriol. 182, 4811–4821 (2000).
Sory, M. P., Boland, A., Lambermont, I. & Cornelis, G. R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc. Natl Acad. Sci. USA 92, 11998–12002 (1995).
Grosdent, N., Maridonneau-Parini, I., Sory, M. P. & Cornelis, G. R. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 70, 4165–4176 (2002).
Andersson, K., Magnusson, K. E., Majeed, M., Stendahl, O. & Fallman, M. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67, 2567–2574 (1999).
Zhang, Z. Y. et al. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267, 23759–23766 (1992).
Guan, K. L. & Dixon, J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 266, 17026–17030 (1991).
Wattiau, P., Bernier, B., Deslee, P., Michiels, T. & Cornelis, G. R. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl Acad. Sci. USA 91, 10493–10497 (1994).
Black, D. S., Montagna, L. G., Zitsmann, S. & Bliska, J. B. Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol. Microbiol. 29, 1263–1274 (1998).
Montagna, L. G., Ivanov, M. I. & Bliska, J. B. Identification of residues in the N-terminal domain of the Yersinia tyrosine phosphatase that are critical for substrate recognition. J. Biol. Chem. 276, 5005–5011 (2001).
Evdokimov, A. G., Tropea, J. E., Routzahn, K. M., Copeland, T. D. & Waugh, D. S. Structure of the N-terminal domain of Yersinia pestis YopH at 2.0 Å resolution. Acta Crystallogr. D. Biol. Crystallogr. 57, 793–799 (2001).
Smith, C. L., Khandelwal, P., Keliikuli, K., Zuiderweg, E. R. & Saper, M. A. Structure of the type III secretion and substrate-binding domain of Yersinia YopH phosphatase. Mol. Microbiol. 42, 967–979 (2001).
Hamid, N. et al. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27, 231–242 (1999).
Persson, C. et al. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33, 828–838 (1999).
Ridley, A. J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477 (2001).
Von Pawel-Rammingen, U. et al. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36, 737–748 (2000).
Sorg, I., Goehring, U. M., Aktories, K. & Schmidt, G. Recombinant Yersinia YopT leads to uncoupling of RhoA–effector interaction. Infect. Immun. 69, 7535–7543 (2001).
Boquet, P. Small GTP binding proteins and bacterial virulence. Microbes Infect. 2, 837–843 (2000).
Galyov, E. E., Hakansson, S., Forsberg, A. & Wolf-Watz, H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361, 730–732 (1993).
Dukuzumuremyi, J. M. et al. The Yersinia protein kinase A is a host factor inducible RhoA/Rac-binding virulence factor. J. Biol. Chem. 275, 35281–35290 (2000).
Juris, S. J., Rudolph, A. E., Huddler, D., Orth, K. & Dixon, J. E. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl Acad. Sci. USA 97, 9431–9436 (2000).
Monack, D. M., Mecsas, J., Bouley, D. & Falkow, S. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice. J. Exp. Med. 188, 2127–2137 (1998).
Galyov, E. E., Hakansson, S. & Wolf-Watz, H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J. Bacteriol. 176, 4543–4548 (1994).
Straley, S. C. & Bowmer, W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect. Immun. 51, 445–454 (1986).
Ruckdeschel, K. et al. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J. Exp. Med. 187, 1069–1079 (1998).
Orth, K. et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285, 1920–1923 (1999).
Meijer, L. K., Schesser, K., Wolf-Watz, H., Sassone-Corsi, P. & Pettersson, S. The bacterial protein YopJ abrogates multiple signal transduction pathways that converge on the transcription factor CREB. Cell Microbiol. 2, 231–238 (2000).
Sauvonnet, N., Pradet-Balade, B., Garcia-Sanz, J. A. & Cornelis, G. R. Regulation of mRNA expression in macrophages following Yersinia enterocolitica infection: role of different Yop effectors. J. Biol. Chem. 277, 25133–25142 (2002).
Mills, S. D. et al. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl Acad. Sci. USA 94, 12638–12643 (1997).
Monack, D. M., Mecsas, J., Ghori, N. & Falkow, S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl Acad. Sci. USA 94, 10385–10390 (1997).
Denecker, G. et al. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276, 19706–19714 (2001).
Ruckdeschel, K. et al. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-κB pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166, 1823–1831 (2001).
Orth, K. et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290, 1594–1597 (2000).
Palframan, R. T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Evdokimov, A. G., Anderson, D. E., Routzahn, K. M. & Waugh, D. S. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 312, 807–821 (2001).
Leung, K. Y., Reisner, B. S. & Straley, S. C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect. Immun. 58, 3262–3271 (1990).
Mulder, B., Michiels, T., Simonet, M., Sory, M. P. & Cornelis, G. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect. Immun. 57, 2534–2541 (1989).
Burrows, T. W. & Bacon, G. A. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br. J. Exp. Pathol. 37, 481–493 (1956).
Skrzypek, E. & Straley, S. C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177, 2530–2542 (1995).
Nakajima, R., Motin, V. L. & Brubaker, R. R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63, 3021–3029 (1995).
Sing, A., Roggenkamp, A., Geiger, A. M. & Heesemann, J. Yersinia enterocolitica evasion of the host innate immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J Immunol. 168, 1315–1321 (2002).
Foultier, B., Troisfontaines, P., Müller, S., Opperdoes, F. & Cornelis, G. R. Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogeny analysis of type III secretion systems. J. Mol. Evol. 55, 37–51 (2002).
Robins-Browne, R. M. & Prpic, J. K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect. Immun. 47, 774–779 (1985).
Rakin, A. & Heesemann, J. Yersinia bactin/pesticin receptor: a component of an iron uptake system of highly pathogenic Yersinia. Contrib. Microbiol. Immunol. 13, 244–247 (1995).
Parkhill, J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527 (2001).
Haller, J. C., Carlson, S., Pederson, K. J. & Pierson, D. E. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36, 1436–1446 (2000).
Aizawa, S. I. Bacterial flagella and type III secretion systems. FEMS Microbiol. Lett. 202, 157–164 (2001).
Macnab, R. M. Microbiology. Action at a distance — bacterial flagellar assembly. Science 290, 2086–2087 (2000).
Russel, M. Phage assembly: a paradigm for bacterial virulence factor export? Science 265, 612–614 (1994).
Michiels, T., Wattiau, P., Brasseur, R., Ruysschaert, J. M. & Cornelis, G. Secretion of Yop proteins by Yersiniae. Infect. Immun. 58, 2840–2849 (1990).Demonstration that Yops are secreted by a new secretion pathway, now called 'type III'.
Anderson, D. M. & Schneewind, O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science 278, 1140–1143 (1997).Presents the mRNA secretion signal hypothesis.
Anderson, D. M. & Schneewind, O. Type III machines of Gram-negative pathogens: injecting virulence factors into host cells and more. Curr. Opin. Microbiol. 2, 18–24 (1999).
Lloyd, S. A., Norman, M., Rosqvist, R. & Wolf-Watz, H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol. Microbiol. 39, 520–531 (2001).
Ramamurthi, K. S. & Schneewind, O. Yersinia enterocolitica type III secretion: mutational analysis of the yopQ secretion signal. J. Bacteriol. 184, 3321–3328 (2002).
Wattiau, P. & Cornelis, G. R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in Ohe secretion of YopE. Mol. Microbiol. 8, 123–131 (1993).First description of a chaperone of the 'type III family'.
Menard, R., Sansonetti, P., Parsot, C. & Vasselon, T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79, 515–525 (1994).
Birtalan, S. C., Phillips, R. M. & Ghosh, P. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 9, 971–980 (2002).
Feldman, M. F., Müller, S., Wüest, E. & Cornelis, G. R. SycE allows secretion of YopE–DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. (in the press).
Birtalan, S. & Ghosh, P. Structure of the Yersinia type III secretory system chaperone SycE. Nature Struct. Biol. 8, 974–978 (2001).
Acknowledgements
I am grateful to all the members of my group for continuous crucial and challenging discussions. I thank L. Journet for supplying Figure 1b, P. Troisfontaines for assistance in preparing Box 1 and M. Feldmann for assistance in preparing the Box 5 figure. I apologize to my collegues whose excellent work could not be cited because of the lack of space. My laboratory in Brussels was supported by the Belgian Fonds National de la Recherche Scientifique Médicale, the Direction Générale de la Recherche Scientifique-Communauté Française de Belgique, and by two European Union TMR networks. In Basel, my laboratory is supported by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Supplementary information
Movie 1 | Phagocytosis of wild-type Yersinia enterocolitica E40 by macrophages transfected with green fluorescent protein–actin.
The multiplicity of infection is 50. On the left, fluorescence microscopy shows the dynamics of actin underneath the bacteria. On the right, the dynamics of actin underneath the bacteria are shown by phase-contrast microscopy. 120 frames were recorded at a speed of one frame every 5 seconds. Two frames are played per second, so one minute of movie represents 10 minutes of real time. You can see that every phagocytic event or attempt involves the formation of a phagocytic 'cup', which is made of fluorescent actin. It is difficult to see from the movie whether every 'cup' engulfs a bacterium or whether some phagocytosis attempts abort. These videos illustrate the speed of phagocytosis and hence the speed of the antiphagocytic response of Yersinia. Note that the actin punctuation develops during the infection, owing to Yop action. This is the work of N. Grosdent and G.R.C., University of Louvain, Belgium, in collaboration with A. Sechi and J. Wehland, Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany.
Movie 2 | Phagocytosis of yscN Yersinia enterocolitica (type III secretion deficient) by macrophages transfected with green fluorescent protein–actin.
The multiplicity of infection is 50. 120 frames were recorded at a speed of one frame every 5 seconds. Two frames are played per second, so one minute of movie represents 10 minutes of real time. Every phagocytic event involves the formation of a phagocytic 'cup', which is made of fluorescent actin. Note the speed of phagocytosis. Most of the phagocytic events take place at one pole of this elongated cell. This is the work of N. Grosdent and G.R.C., University of Louvain, Belgium, in collaboration with A. Sechi and J. Wehland, Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany.
Movie 3 | Destruction of the cytoskeleton of Rat-1 fibroblasts by Yersinia enterocolitica E40.
Rat-1 fibroblasts that are transiently transfected with green fluorescent protein–actin were infected with wild-type Y. enterocolitica E40 at a multiplicity of infection of 50. One frame was taken every minute, and one frame is played per second, so the movie sequence seen is 60 times faster than the real-time sequence of events. You can see the destruction of the cytoskeleton and the rounding of the fibroblasts. This is the work of N. Grosdent and G.R.C., University of Louvain, Belgium, in collaboration with A. Sechi and J. Wehland, Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany.
Related links
Related links
DATABASES
Entrez
LocusLink
Swiss-Prot
FURTHER INFORMATION
Glossary
- EXOTOXINS
-
Bacterial protein toxins that are secreted by pathogenic bacteria and that contribute to infectious disease.
- MACROPHAGES
-
Long-lived bone-marrow-derived cells that are central to the host defence against microbes. Their main functions are phagocytosis, antigen-presentation and the release of inflammatory cytokines.
- POLYMORPHONUCLEAR LEUKOCYTES
-
Short-lived bone-marrow-derived cells with high motility and phagocytic capacities.
- INJECTISOME
-
The organelle responsible for 'secretion' of virulence proteins by the 'type III secretion' mechanism.
- BASAL BODY
-
The basal body of the flagellum is the part that is embedded in the cell surface and that is in the bacterial cytoplasm. It consists of a rod, a set of four thin rings (L, P, S and M), and a large ring (C) that contains the flagellin-export apparatus.
- PEPTIDOGLYCAN
-
Peptidoglycan is the rigid, shape-determining complex polymer that forms the cell wall of bacteria. It is made of chains of heteropolysaccharides linked by tetrapeptides.
- FLAGELLUM
-
The locomotive organelle of bacteria. It consists of a basal body and a long hollow filament that is rotated by a molecular motor.
- F0F1 PROTON TRANSLOCASE
-
A large and complex enzyme in the mitochondrial inner membrane that catalyses the synthesis of ATP, which is driven by a flow of protons.
- PSYCHROPHILIC BACTERIA
-
Bacteria that can grow at 4 °C.
- CHAPERONES
-
Several families of proteins, known as molecular chaperones, that assist nascent proteins in their folding or prevent premature or illicit associations with other proteins, folded or partially folded. Some chaperones, such as those involved in type III secretion, are small (∼15 kDa) proteins without any ATP-binding site, whereas others, known as chaperonins, form large heteropolymeric cylinders and consume ATP.
- ADHESINS
-
Bacterial proteins that promote adherence to host-cell membranes. Some are simply anchored in the bacterial membrane, whereas others are placed at the tips of pili.
- NECROSIS
-
Death in response to cell or tissue damage, which ends in the release of the intracellular content and the onset of inflammation.
- STRESS FIBRES
-
Long axial bundles of actin microfilaments that run along the entire length of the cell.
- PHOSPHOTYROSINE-BINDING (PTB) DOMAINS
-
Domains that bind phosphotyrosine residues and allow signalling interactions.
- SH2 DOMAINS
-
Src-homology 2 domains are PTB domains that bind to phosphotyrosine residues, such as those that are found in activated receptor- or cytoplasmic-tyrosine kinases, and are involved in signalling processes.
- RhoA, Rac AND Cdc42
-
Monomeric GTPases that are involved in the control of actin polymerization/depolymerization.
- OPSONIZATION
-
The process by which IgG or complement C3b molecules bind to and coat particles, which enhances the efficiency of phagocytosis.
- APOPTOSIS
-
Programmed cell death governed by complex signalling pathways and marked by a well-defined sequence of morphological changes, resulting in small bodies that are phagocytosed by other cells.
Rights and permissions
About this article
Cite this article
Cornelis, G. The Yersinia Ysc–Yop 'Type III' weaponry. Nat Rev Mol Cell Biol 3, 742–753 (2002). https://doi.org/10.1038/nrm932
Issue Date:
DOI: https://doi.org/10.1038/nrm932
This article is cited by
-
Exploring resveratrol dimers as virulence blocking agents – Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa
Scientific Reports (2020)
-
LITESEC-T3SS - Light-controlled protein delivery into eukaryotic cells with high spatial and temporal resolution
Nature Communications (2020)
-
Interactions between Yersinia pestis V-antigen (LcrV) and human Toll-like receptor 2 (TLR2) in a modelled protein complex and potential mechanistic insights
BMC Immunology (2019)
-
Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics
Genes & Immunity (2019)
-
Investigation of the anti-apoptotic activity of ozone therapy in rainbow trout macrophages infected with Yersinia ruckeri
Aquaculture International (2019)