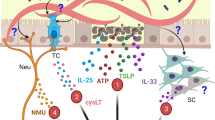
Key Points
-
Infection with large metazoan parasites (helminths) typically induces a type 2 immune response. Redundancy within the immune system, as well as extensive dialogue between cells of the immune system and non-immune cells, generates enormous complexity.
-
The central player in type 2 immunity is the CD4+ T helper 2 (TH2) cell, which produces a broad range of cytokines, including interleukin-4 (IL-4) and IL-13, which act on target cells expressing the IL-4 receptor α-chain. Target cells include most cells of the immune system but also local tissue cells such as epithelial cells that line mucosal surfaces.
-
Cells of the innate immune system, such as the recently described 'innate helper cells', can also produce type 2 cytokines. These cells function as effectors during the early stages of infection, but additionally create an environment that favours the induction of TH2-type responses.
-
TH2-type responses are initiated by alarm signals from epithelial cells, as well as by specific recognition of helminth products. A strict requirement for dendritic cells in this process has been established.
-
In addition to killing or expelling helminth parasites, type 2 immune responses contribute to rapid tissue repair, and this sometimes leads to fibrosis-related pathology. Many facets of type 2 immunity are consistent with evolutionary origins in wound-healing pathways, a reflection of the capacity of helminth parasites to damage tissue through migration and feeding.
-
T cell dynamics change over time, and TH2-type responses often decline during chronic helminth infection. Regulatory pathways, including regulatory T cells, restrain pathology and immune responses during infection, and some helminths are able to actively induce the expansion of regulatory populations.
-
Because mammals evolved in the presence of chronic infection, their immune systems may have compensated for the immune dampening effects of helminths. If so, over-reactive responses to innocuous antigens in the absence of infection may contribute to autoimmune disease and allergy.
Abstract
The vertebrate immune system has evolved in concert with a broad range of infectious agents, including ubiquitous helminth (worm) parasites. The constant pressure of helminth infections has been a powerful force in shaping not only how immunity is initiated and maintained, but also how the body self-regulates and controls untoward immune responses to minimize overall harm. In this Review, we discuss recent advances in defining the immune cell types and molecules that are mobilized in response to helminth infection. Finally, we more broadly consider how these immunological players are blended and regulated in order to accommodate persistent infection or to mount a vigorous protective response and achieve sterile immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koyasu, S., Moro, K., Tanabe, M. & Takeuchi, T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv. Immunol. 108, 21–44 (2010).
Saenz, S. A., Noti, M. & Artis, D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 31, 407–413 (2010).
Wojciechowski, W. et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 30, 421–433 (2009).
Paul, W. E. & Zhu, J. How are TH2-type immune responses initiated and amplified? Nature Rev. Immunol. 10, 225–235 (2010).
Taylor, M. D. et al. Early recruitment of natural CD4+ Foxp3+ TReg cells by infective larvae determines the outcome of filarial infection. Eur. J. Immunol. 39, 192–206 (2009).
Finkelman, F. D. et al. Interleukin-4 and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201, 139–155 (2004).
Jenkins, S. J. & Allen, J. E. Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes. J. Biomed. Biotechnol. 2010, 262–609 (2010).
Anthony, R. M., Rutitzky, L. I., Urban, J. F., Stadecker, M. J. & Gause, W. C. Protective immune mechanisms in helminth infection. Nature Rev. Immunol. 7, 975–987 (2007).
Neill, D. R. & Mc Kenzie, A. N. J. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 27, 214–221 (2011).
Vignali, D. A. et al. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology 67, 466–472 (1989).
Katona, I. M., Urban, J. F. & Finkelman, F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J. Immunol. 140, 3206–3211 (1988).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R. M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001). This study developed and used a powerful method for tracking IL-4-producing cells.
Urban, J. F. et al. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998).
Zhu, J. et al. Conditional deletion of Gata3 shows its essential function in TH1-TH2 responses. Nature Immunol. 5, 1157–1165 (2004).
Voehringer, D., Reese, T. A., Huang, X., Shinkai, K. & Locksley, R. M. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203, 1435–1446 (2006).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Saenz, S. A. et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464, 1362–1366 (2010). References 16–19 defined the phenotype and functional role of innate helper cells in gastrointestinal nematode infection.
Saenz, S. A., Taylor, B. C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Hasnain, S. Z. et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 208, 893–900 (2011).
Herbert, D. R. et al. Intestinal epithelial cell secretion of RELM-α protects against gastrointestinal worm infection. J. Exp. Med. 206, 2947–2957 (2009). This study demonstrates that an innate epithelial cell product exerts direct anti-parasite effects on gut nematodes.
Artis, D. et al. RELMα/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl Acad. Sci. USA 101, 13596–13600 (2004).
Akiho, H., Blennerhassett, P., Deng, Y. & Collins, S. M. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G226–G232 (2002).
Cliffe, L. J. et al. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308, 1463–1465 (2005). The discovery of an important new T H 2 cell-mediated mechanism of protection against parasites in the GI tract.
McDermott, J. R. et al. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc. Natl Acad. Sci. USA 100, 7761–7766 (2003).
Sasaki, Y. et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J. Exp. Med. 202, 607–616 (2005).
Anthony, R. M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nature Med. 12, 955–960 (2006). The first description of an anti-parasite role for alternatively activated macrophages.
Min, B. et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 200, 507–517 (2004).
Mitre, E., Taylor, R. T., Kubofcik, J. & Nutman, T. B. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J. Immunol. 172, 2439–2445 (2004).
Karasuyama, H., Wada, T., Yoshikawa, S. & Obata, K. Emerging roles of basophils in protective immunity against parasites. Trends Immunol. 32, 125–130 (2011).
Ohnmacht, C. et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33, 364–374 (2010). This study defines the roles, and the boundaries, of basophils during tissue and gut nematode infection.
Kim, S. et al. Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Sabin, E. A., Kopf, M. A. & Pearce, E. J. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184, 1871–1878 (1996).
Knott, M. L. et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int. J. Parasitol. 37, 1367–1378 (2007).
Spencer, L. A. & Weller, P. F. Eosinophils and Th2 immunity: contemporary insights. Immunol. Cell Biol. 88, 250–256 (2010).
Padigel, U. M. et al. Signaling through Gαi2 protein is required for recruitment of neutrophils for antibody-mediated elimination of larval Strongyloides stercoralis in mice. J. Leukoc. Biol. 81, 1120–1126 (2007).
Al-Qaoud, K. M. et al. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 12, 899–908 (2000).
Harvie, M. et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect. Immun. 78, 3753–3762 (2010).
Nair, M. G. et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect. Immun. 73, 385–394 (2005).
Reece, J. J., Siracusa, M. C. & Scott, A. L. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect. Immun. 74, 4970–4981 (2006).
Satoguina, J. S., Weyand, E., Larbi, J. & Hoerauf, A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J. Immunol. 174, 4718–4726 (2005).
Harris, N. & Gause, W. C. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 32, 80–88 (2011).
McCoy, K. D. et al. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4, 362–373 (2008). This study defines the settings in which antibodies function in an anti-nematode role, including the importance of polyclonal immunoglobulins.
Dunne, D. W. et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22, 1483–1494 (1992).
Kooyman, F. et al. Protection in lambs vaccinated with Haemonchus contortus antigens is age related, and correlates with IgE rather than IgG1 antibody. Parasite Immunol. 22, 13–20 (2000).
de Andres, B. et al. Lack of Fc-ɛ receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood 89, 3826–3836 (1997).
Capron, M. & Capron, A. Immunoglobulin E and effector cells in schistosomiasis. Science 264, 1876–1877 (1994).
Abraham, D. et al. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect. Immun. 72, 810–817 (2004).
Maizels, R. M. & Yazdanbakhsh, M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Rev. Immunol. 3, 733–744 (2003).
Platts-Mills, T. A. E., Woodfolk, J. A., Erwin, E. A. & Aalberse, R. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer Semin. Immunopathol. 25, 271–279 (2004).
Rodríguez-Sosa, M. et al. A STAT4-dependent Th1 response is required for resistance to the helminth parasite Taenia crassiceps. Infect. Immun. 72, 4552–4560 (2004).
Wynn, T. A. et al. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J. Immunol. 157, 4068–4078 (1996).
Dessein, A. et al. Interleukin-13 in the skin and interferon-γ in the liver are key players in immune protection in human schistosomiasis. Immunol. Rev. 201, 180–190 (2004).
Díaz, A. & Allen, J. E. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur. J. Immunol. 37, 3319–3326 (2007).
James, S. L. & Glaven, J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 143, 4208–4212 (1989).
Allen, J. E. & Wynn, T. A. Evolution of Th2 immunity: a rapid repair response to the tissue destructive pathogens. PLoS Pathog. 7, e1002003 (2011).
Miyake, K., Tanaka, T. & McNeil, P. L. Disruption-induced mucus secretion: repair and protection. PLoS Biol. 4, e276 (2006).
Enoksson, M. et al. Mast cells as sensors of cell injury through IL-33 recognition. J. Immunol. 186, 2523–2528 (2011).
Lee, J. J., Jacobsen, E. A., McGarry, M. P., Schleimer, R. P. & Lee, N. A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575 (2010).
Eming, S. A., Krieg, T. & Davidson, J. M. Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 (2007).
Redente, E. F. et al. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J. Leukoc. Biol. 88, 159–168 (2010).
Profet, M. The function of allergy: immunological defense against toxins. Q. Rev. Biol. 66, 23–62 (1991).
Skugor, S., Glover, K. A., Nilsen, F. & Krasnov, A. Local and systemic gene expression responses of Atlantic salmon (Salmo salar L.) to infection with the salmon louse (Lepeophtheirus salmonis). BMC Genomics 9, 498 (2008).
Seno, H. et al. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc. Natl Acad. Sci. USA 106, 256–261 (2009).
Turnbull, I. R. et al. Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 177, 3520–3524 (2006).
Kühn, H. & O'Donnell, V. B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 45, 334–356 (2006).
Nair, M. G. et al. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937–952 (2009).
Pesce, J. T. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371 (2009). This study provides evidence that arginase 1, a T H 2-type effector molecule, may have different functions depending on the cell type that produces it.
Pesce, J. T. et al. Retnla (Relmα/Fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 5, e1000393 (2009).
Edwards, A. D. et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169, 3652–3660 (2002).
Robinson, M. J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206, 2037–2051 (2009).
Balic, A., Harcus, Y., Holland, M. J. & Maizels, R. M. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur. J. Immunol. 34, 3047–3059 (2004).
Cervi, L., Mac Donald, A. S., Kane, C., Dzierszinski, F. & Pearce, E. J. Dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 172, 2016–2020 (2004).
Robinson, M. W., Hutchinson, A. T., Donnelly, S. & Dalton, J. P. Worm secretory molecules are causing alarm. Trends Parasitol. 26, 371–372 (2010).
Loke, P. et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179, 3926–3936 (2007). This paper reports that IL-4Rα responses are an innate response to injury and has implications for T H 2-type immunity and wound repair.
Zhao, A. et al. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J. Immunol. 185, 6921–6929 (2010).
Kouzaki, H., O'Grady, S. M., Lawrence, C. B. & Kita, H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J. Immunol. 183, 1427–1434 (2009).
Lüthi, A. U. et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 31, 84–98 (2009).
Tawill, S., Le Goff, L., Ali, F., Blaxter, M. & Allen, J. E. Both free-living and parasitic nematodes induce a characteristic Th2 response that is dependent on the presence of intact glycans. Infect. Immun. 72, 398–407 (2004).
Everts, B. et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. J. Exp. Med. 206, 1673–1680 (2009).
Hewitson, J. P., Grainger, J. R. & Maizels, R. M. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 167, 1–11 (2009).
Jankovic, D. et al. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10−/– setting. Immunity 16, 429–439 (2002).
Steinfelder, S. et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1). J. Exp. Med. 206, 1681–1690 (2009). Together with reference 81, this study identifies an individual T H 2-type response-driving molecule from schistosome eggs that acts via DCs.
van Liempt, E. et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 44, 2605–2615 (2007).
Ritter, M. et al. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl Acad. Sci. USA 107, 20459–20464 (2010).
Perrigoue, J. G. et al. MHC class II-dependent basophil–CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nature Immunol. 10, 697–705 (2009).
Phythian-Adams, A. T. et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J. Exp. Med. 207, 2089–2096 (2010).
MacDonald, A. S. & Pearce, E. J. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J. Immunol. 168, 3127–3130 (2002).
Torrero, M. N., Hübner, M. P., Larson, D., Karasuyama, H. & Mitre, E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J. Immunol. 185, 7426–7434 (2010).
Everts, B., Smits, H. H., Hokke, C. H. & Yazdanbakhsh, M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur. J. Immunol. 40, 1525–1537 (2010).
MacDonald, A. S. & Maizels, R. M. Alarming dendritic cells for Th2 induction. J. Exp. Med. 205, 13–17 (2008).
Horsnell, W. G. C. et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Rα-deficient mice. PLoS Pathog. 3, e1 (2007).
Taylor, B. C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Massacand, J. C. et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl Acad. Sci. USA 106, 13968–13973 (2009).
Segura, M., Su, Z., Piccirillo, C. & Stevenson, M. M. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 37, 1887–1904 (2007).
Mylonas, K. J., Nair, M. G., Prieto-Lafuente, L., Paape, D. & Allen, J. E. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J. Immunol. 182, 3084–3094 (2009).
Loke, P., MacDonald, A. S. & Allen, J. E. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naïve CD4+ T cells. Eur. J. Immunol. 30, 1127–1135 (2000).
Huber, S., Hoffmann, R., Muskens, F. & Voehringer, D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood 116, 3311–3320 (2010).
Terrazas, L. I., Montero, D., Terrazas, C. A., Reyes, J. L. & Rodríguez-Sosa, M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 35, 1349–1358 (2005).
Munder, M. et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105, 2549–2556 (2005).
Babu, S., Kumaraswami, V. & Nutman, T. B. Alternatively activated and immunoregulatory monocytes in human filarial infections. J. Infect. Dis. 199, 1827–1837 (2009).
Zaretsky, A. G. et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206, 991–999 (2009).
Veldhoen, M. et al. Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunol. 9, 1341–1346 (2008).
Zhou, L., Chong, M. M. W. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Balic, A., Harcus, Y. M., Taylor, M. D., Brombacher, F. & Maizels, R. M. IL-4R signaling is required to induce IL-10 for the establishment of Th2 dominance. Int. Immunol. 18, 1421–1431 (2006).
Helmby, H. & Grencis, R. K. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur. J. Immunol. 33, 2382–2390 (2003).
Rutitzky, L. I. et al. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 180, 2486–2495 (2008).
Pedras-Vasconcelos, J. A. & Pearce, E. J. Type 1 CD8+ T cell responses during infection with the helminth Schistosoma mansoni. J. Immunol. 157, 3046–3053 (1996).
Mallevaey, T. et al. Activation of invariant NKT cells by the helminth parasite Schistosoma mansoni. J. Immunol. 176, 2476–2485 (2006).
Boros, D. L., Pelley, R. P. & Warren, K. S. Spontaneous modulation of granulomatous hypersensitivity in schistosomiasis mansoni. J. Immunol. 114, 1437–1441 (1975).
Sartono, E., Kruize, Y. C., Kurniawan, A., Maizels, R. M. & Yazdanbakhsh, M. Depression of antigen-specific interleukin-5 and interferon-γ responses in human lymphatic filariasis as a function of clinical status and age. J. Infect. Dis. 175, 1276–1280 (1997).
Taylor, M. D. et al. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174, 4924–4933 (2005). This was the first description of the requirement for T Reg cells to maintain susceptibility to nematode infection.
Taylor, M. D. et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 179, 4626–4634 (2007).
Grogan, J. L., Kremsner, P. G., Deelder, A. M. & Yazdanbakhsh, M. Antigen-specific proliferation and interferon-γ and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 177, 1433–1437 (1998).
Taylor, J. J., Krawczyk, C. M., Mohrs, M. & Pearce, E. J. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J. Clin. Invest. 119, 1019–1028 (2009).
Smith, P. et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J. Immunol. 173, 1240–1248 (2004).
Finney, C. A. M., Taylor, M. D., Wilson, M. S. & Maizels, R. M. Expansion and activation of CD4+CD25+ regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 37, 1874–1886 (2007).
Rausch, S. et al. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 76, 1908–1919 (2008).
Fleming, J. & Fabry, Z. The hygiene hypothesis and multiple sclerosis. Ann. Neurol. 61, 85–89 (2007).
Elliott, D. E., Summers, R. W. & Weinstock, J. V. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 37, 457–464 (2007).
Maizels, R. M. Infections and allergy — helminths, hygiene and host immune regulation. Curr. Opin. Immunol. 17, 656–661 (2005).
Fallon, P. G. & Mangan, N. E. Suppression of TH2-type allergic reactions by helminth infection. Nature Rev. Immunol. 7, 220–230 (2007).
McSorley, H. J., Harcus, Y. M., Murray, J., Taylor, M. D. & Maizels, R. M. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J. Immunol. 181, 6456–6466 (2008).
Grainger, J. R. et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med. 207, 2331–2341 (2010). This study demonstrates that expansion of TReg cell populations in helminth infection can be driven by parasite products exploiting the TGF-β pathway.
Babu, S. et al. Filarial lymphedema is characterized by antigen-specific Th1 and Th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl. Trop. Dis. 3, e420 (2009).
Turner, J. D. et al. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 197, 1204–1212 (2008).
Figueiredo, C. A. et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun. 78, 3160–3167 (2010).
Layland, L. E., Rad, R., Wagner, H. & da Costa, C. U. P. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 37, 2174–2184 (2007).
D'Elia, R., Behnke, J. M., Bradley, J. E. & Else, K. J. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J. Immunol. 182, 2340–2348 (2009).
Zaccone, P. et al. Schistosoma mansoni egg antigens induce TReg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 39, 1098–1107 (2009).
van der Kleij, D. et al. A novel host–parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
Metenou, S. et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 184, 5375–5382 (2010).
Correale, J., Farez, M. & Razzitte, G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann. Neurol. 64, 187–199 (2008).
Mangan, N. E. et al. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173, 6346–6356 (2004).
Smits, H. H. et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J. Allergy Clin. Immunol. 120, 932–940 (2007).
Wilson, M. S. et al. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur. J. Immunol. 40, 1682–1696 (2010).
Kreider, T., Anthony, R. M., Urban, J. F. & Gause, W. C. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19, 448–453 (2007).
Wong, D. T. et al. Eosinophils from patients with blood eosinophilia express transforming growth factor-β1. Blood 78, 2702–2707 (1991).
Humbles, A. A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004).
Behnke, J. M., Barnard, C. J. & Wakelin, D. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int. J. Parasitol. 22, 861–907 (1992).
Graham, A. L., Allen, J. E. & Read, A. F. Evolutionary causes and consequences of immunopathology. Annu. Rev. Ecol. Evol. Syst. 36, 373–397 (2005).
Mentink-Kane, M. M. & Wynn, T. A. Opposing roles for IL-13 and IL-13 receptor α2 in health and disease. Immunol. Rev. 202, 191–202 (2004).
Hoffmann, K. F., Wynn, T. A. & Dunne, D. W. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv. Parasitol. 52, 265–307 (2002).
Hoffmann, K. F., James, S. L., Cheever, A. W. & Wynn, T. A. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163, 927–938 (1999).
Schneider, D. S. & Ayres, J. S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Rev. Immunol. 8, 889–895 (2008).
Loke, P. et al. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3, 7 (2002).
Gordon, S. Alternative activation of macrophages. Nature Rev. Immunol. 3, 23–35 (2003).
Hesse, M. et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 (2001).
Choi, B. et al. Differential impact of L-arginine deprivation on the activation and effector functions of T cells and macrophages. J. Leukoc. Biol. 85, 268–277 (2009).
Teng, X., Li, D., Champion, H. C. & Johns, R. A. FIZZ1/RELMα, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ. Res. 92, 1065–1067 (2003).
Yamaji-Kegan, K. et al. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J. Immunol. 185, 5539–5548 (2010).
Liu, T. et al. FIZZ1 stimulation of myofibroblast differentiation. Am. J. Pathol. 164, 1315–1326 (2004).
Hung, S., Chang, A. C., Kato, I. & Chang, N. A. Transient expression of Ym1, a heparin-binding lectin, during developmental hematopoiesis and inflammation. J. Leukoc. Biol. 72, 72–82 (2002).
Arora, M. et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc. Natl Acad. Sci. USA 103, 7777–7782 (2006).
Cai, Y., Kumar, R. K., Zhou, J., Foster, P. S. & Webb, D. C. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J. Immunol. 182, 5393–5399 (2009).
Weaver, C. T. & Hatton, R. D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nature Rev. Immunol. 9, 883–889 (2009).
Maizels, R. M. Parasite immunomodulation and polymorphisms of the immune system. J. Biol. 8, 62 (2009).
Fumagalli, M. et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 206, 1395–1408 (2009). This study provides evidence that populations exposed to a greater range of different helminth parasites have greater immune gene diversity, and higher frequencies of certain alleles linked to autoimmunity.
Jackson, J. A. et al. Immunomodulatory parasites and Toll-like receptor-mediated tumour necrosis factor-α responsiveness in wild mammals. BMC Biol. 7, 16 (2009).
Graham, A. L. et al. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330, 662–665 (2010).
Lamb, E. W. et al. Blood fluke exploitation of non-cognate CD4+ T cell help to facilitate parasite development. PLoS Pathog. 6, e1000892 (2010).
Karanja, D. M., Colley, D. G., Nahlen, B. L., Ouma, J. H. & Secor, W. E. Studies on schistosomiasis in western Kenya: I. Evidence for immune-facilitated excretion of schistosome eggs from patients with Schistosoma mansoni and human immunodeficiency virus coinfections. Am. J. Trop. Med. Hyg. 56, 515–521 (1997).
Lamb, E. W. et al. Conservation of CD4+ T cell-dependent developmental mechanisms in the blood fluke pathogens of humans. Int. J. Parasitol. 37, 405–415 (2007).
Babayan, S. A., Read, A. F., Lawrence, R. A., Bain, O. & Allen, J. E. Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol. 8, e1000525 (2010).
Fabre, V. et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J. Immunol. 182, 1577–1583 (2009).
Telfer, S. et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246 (2010).
Hayes, K. S. et al. Exploitation of the intestinal microflora by the parasitic nematode Trichuris muris. Science 328, 1391–1394 (2010).
LaPorte, S. L. et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 132, 259–272 (2008).
Specht, S. et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect. Immun. 74, 5236–5243 (2006).
Dessaint, J. P. & Capron, A. Fcɛ receptor II-positive macrophages and platelets: potent effector cells in allergy and defence against helminth parasites. Springer Semin. Immunopathol. 12, 349–363 (1990).
Acknowledgements
The authors gratefully acknowledge funding support from Asthma UK, the UK Medical Research Council and the Wellcome Trust (to J.E.A. and R.M.M.), the European Commission (to J.E.A.) and the American Asthma Foundation (to R.M.M.). We thank the members of our laboratories for the extensive discussions and interactions that have helped develop many of the concepts in this Review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Innate helper cell
-
A lymphoid cell that lacks antigen-specific receptors (such as B or T cell receptors) but that has the capacity to make cytokines associated with T helper (TH) cells (for example, the TH2-type cytokines interleukin-4 (IL-4), IL-5 and IL-13) in response to innate 'alarm' cytokines, such as IL-25 and IL-33.
- Non-B, non-T cells
-
(NBNT cells). Cells that are distinct from immunoglobulin- or T cell receptor-bearing lymphocytes, basophils, eosinophils, mast cells and natural killer T cells and can produce T helper 2 (TH2)-type cytokines.
- Tight junctions
-
A tight junction is a belt-like region of adhesion between adjacent epithelial or endothelial cells that regulates paracellular flux. Tight-junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludens proteins.
- Recombination activating gene (RAG)-deficient mice
-
Recombination activating genes are involved in creating the double strand DNA breaks necessary for producing the rearranged gene segments that encode the complete protein chains of T cell and B cell receptors. Mice that are deficient for these genes fail to produce B and T cells owing to a developmental block in the gene rearrangement that is necessary for antigen receptor expression.
- Anergy
-
A state of unresponsiveness that is sometimes observed in T and B cells that are chronically stimulated or are stimulated through the antigen receptor in the absence of co-stimulatory signals.
- Regulatory B cells
-
Populations of B cells that downregulate immune responses. These cells are most often associated with production of the immunosuppressive cytokine interleukin-10.
- T regulatory type 1 cells
-
(TR1 cells). A subset of CD4+ regulatory T cells that secrete high levels of interleukin-10 (IL-10) and downregulate T helper 1 (TH1) and TH2 cell responses in vitro and in vivo by a contact-independent mechanism mediated by the secretion of soluble IL-10 and transforming growth factor-β.
- Hygiene hypothesis
-
This hypothesis originally proposed that the increased incidence of atopic diseases in westernized countries was a consequence of living in an overly clean environment, with reduced bacterial exposure predisposing to increased T helper 2 (TH2)-type allergic responses to harmless antigens. More recently, it has been proposed that an absence of exposure to a broader range of pathogens, including helminths, may weaken the immunoregulatory controls that exist to restrain allergy and autoimmune disease.
Rights and permissions
About this article
Cite this article
Allen, J., Maizels, R. Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11, 375–388 (2011). https://doi.org/10.1038/nri2992
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2992
This article is cited by
-
Mechanismus der allergischen Sensibilisierung und wozu hat ihn die Evolution eigentlich geschaffen?
Deutsche Zeitschrift für Akupunktur (2024)
-
Explanatory integration and integrated explanations in Darwinian medicine and evolutionary medicine
Theoretical Medicine and Bioethics (2023)
-
Echinococcus granulosus sensu stricto and antigen B may decrease inflammatory bowel disease through regulation of M1/2 polarization
Parasites & Vectors (2022)
-
Enteric neuroimmune interactions coordinate intestinal responses in health and disease
Mucosal Immunology (2022)
-
Effects of helminths on the human immune response and the microbiome
Mucosal Immunology (2022)