Key Points
-
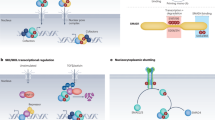
WNT-mediated signalling involves the binding of WNT proteins to their receptor–co-receptor complexes, frizzled–LRP (low-density-lipoprotein-receptor-related protein), which leads (by several steps) to the accumulation of β-catenin and its translocation to the nucleus. In the nucleus, β-catenin forms a bipartite transcription-factor complex with T-cell factor (TCF) to activate target genes.
-
For haematopoietic stem cells (HSCs), the WNT-signalling pathway provides signals for self-renewal proliferation.
-
In the thymus, WNT signalling provides proliferative signals to double-negative thymocytes.
-
TCF1-deficient mice have severe defects in immature thymocyte development. LEF1 (lymphocyte-enhancer-binding factor 1) partially compensates for TCF1.
-
WNT-deficient mice have mild thymic phenotypes, probably because of molecular redundancy. Inhibition of WNT signalling by other means (for example, using soluble frizzled ectodomains) results in more pronounced inhibition of T-cell development.
-
WNT signals are also implicated in the survival of double-positive thymocytes and in the development of thymic epithelial cells.
-
Studies of β-catenin-deficient mice have generated conflicting results regarding thymic phenotype.
-
WNT proteins also provide proliferative signals to pro-B cells.
-
Dysregulated WNT signalling is implicated in various haematological malignancies.
Abstract
The evolutionarily conserved WNT-signalling pathway has pivotal roles during the development of many organ systems, and dysregulated WNT signalling is a key factor in the initiation of various tumours. Recent studies have implicated a role for WNT signal transduction at several stages of lymphocyte development and in the self-renewal of haematopoietic stem cells. Here, we outline new insights into the WNT-signalling pathway, review its role in the self-renewal of haematopoietic stem cells and in the development of T and B cells, and discuss controversies and future developments with regard to WNT signalling in the thymus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nusse, R. & Varmus, H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31, 99–109 (1982).
van Noort, M. & Clevers, H. TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev. Biol. 244, 1–8 (2002).
Bhardwaj, G. et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nature Immunol. 2, 172–180 (2001).
Bhatia, M. et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J. Exp. Med. 189, 1139–1148 (1999).
Varnum-Finney, B. et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 91, 4084–4091 (1998).
Tamai, K. et al. LDL-receptor-related proteins in Wnt signal transduction. Nature 407, 530–535 (2000).
Mao, J. et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 (2001).
Mao, B. et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411, 321–325 (2001).
Hsieh, J. C. Specificity of WNT-receptor interactions. Front. Biosci. 9, 1333–1338 (2004).
Malbon, C. C. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front. Biosci. 9, 1048–1058 (2004).
Wang, H. Y. WNT-frizzled signaling via cyclic GMP. Front. Biosci. 9, 1043–1047 (2004).
Kuhl, M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front. Biosci. 9, 967–974 (2004).
Clevers, H. Wnt breakers in colon cancer. Cancer Cell 5, 5–6 (2004).
Wang, H. Y. & Malbon, C. C. Wnt–frizzled signaling to G-protein-coupled effectors. Cell. Mol. Life Sci. 61, 69–75 (2004).
Ozawa, M., Baribault, H. & Kemler, R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 8, 1711–1717 (1989).
Behrens, J. et al. Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596–599 (1998).
Karim, R., Tse, G., Putti, T., Scolyer, R. & Lee, S. The significance of the Wnt pathway in the pathology of human cancers. Pathology 36, 120–128 (2004).
Seto, E. S. & Bellen, H. J. The ins and outs of Wingless signaling. Trends Cell Biol. 14, 45–53 (2004).
Nelson, W. J. & Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 (2004).
van Es, J. H., Barker, N. & Clevers, H. You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr. Opin. Genet. Dev. 13, 28–33 (2003).
Roose, J. et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395, 608–612 (1998).
Barker, N. et al. The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. EMBO J. 20, 4935–4943 (2001).
Takemaru, K. et al. Chibby, a nuclear β-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422, 905–909 (2003).
Daniels, D. L. & Weis, W. I. ICAT inhibits β-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol. Cell 10, 573–584 (2002).
Kramps, T. et al. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear β-catenin–TCF complex. Cell 109, 47–60 (2002).
Townsley, F. M., Thompson, B. & Bienz, M. Pygopus residues required for its binding to Legless are critical for transcription and development. J. Biol. Chem. 279, 5177–5183 (2004).
Townsley, F. M., Cliffe, A. & Bienz, M. Pygopus and Legless target Armadillo/β-catenin to the nucleus to enable its transcriptional co-activator function. Nature Cell Biol. 6, 626–633 (2004). References 25–27 show the importance of LGS and PYGO for transcriptional activation of the β-catenin–TCF complex.
Thompson, B. J. A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes. Curr. Biol. 14, 458–466 (2004).
Austin, T. W., Solar, G. P., Ziegler, F. C., Liem, L. & Matthews, W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood 89, 3624–3635 (1997).
van den Berg, D. J., Sharma, A. K., Bruno, E. & Hoffman, R. Role of members of the Wnt gene family in human hematopoiesis. Blood 92, 3189–3202 (1998).
Murdoch, B. et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc. Natl Acad. Sci. USA 100, 3422–3427 (2003).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003). References 32 and 33 implicate WNT signalling in the self-renewal of HSCs. Reference 33 mainly describes the purification of biologically active recombinant WNT3A protein, using HSC proliferation as a read-out.
Verbeek, S. et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374, 70–74 (1995). This paper describes the two types of TCF1-deficient mice and their thymic phenotypes.
Schilham, M. W. et al. Critical involvement of Tcf-1 in expansion of thymocytes. J. Immunol. 161, 3984–3991 (1998).
Prieve, M. G. & Waterman, M. L. Nuclear localization and formation of β-catenin–lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol. Cell. Biol. 19, 4503–4515 (1999).
Travis, A., Amsterdam, A., Belanger, C. & Grosschedl, R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor-α enhancer function. Genes Dev. 5, 880–894 (1991).
van Genderen, C. et al. Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, 2691–2703 (1994).
Okamura, R. M. et al. Redundant regulation of T cell differentiation and TCRα gene expression by the transcription factors LEF-1 and TCF-1. Immunity 8, 11–20 (1998). This paper describes the thymic phenotype of mice that are deficient in both TCF1 and LEF1.
Galceran, J., Farinas, I., Depew, M. J., Clevers, H. & Grosschedl, R. Wnt3a−/−-like phenotype and limb deficiency in Lef1−/−Tcf1−/− mice. Genes Dev. 13, 709–717 (1999).
Van de Wetering, M., Castrop, J., Korinek, V. & Clevers, H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol. Cell. Biol. 16, 745–752 (1996).
Ioannidis, V., Beermann, F., Clevers, H. & Held, W. The β-catenin–TCF-1 pathway ensures CD4+CD8+ thymocyte survival. Nature Immunol. 2, 691–697 (2001).
Staal, F. J. et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur. J. Immunol. 31, 285–293 (2001).
Pongracz, J., Hare, K., Harman, B., Anderson, G. & Jenkinson, E. J. Thymic epithelial cells provide WNT signals to developing thymocytes. Eur. J. Immunol. 33, 1949–1956 (2003).
Mulroy, T., McMahon, J. A., Burakoff, S. J., McMahon, A. P. & Sen, J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur. J. Immunol. 32, 967–971 (2002).
Hsu, W., Shakya, R. & Costantini, F. Impaired mammary gland and lymphoid development caused by inducible expression of Axin in transgenic mice. J. Cell Biol. 155, 1055–1064 (2001).
Ranheim, E. A. et al. Frizzled 9 knockout mice have abnormal B cell development. Blood 30 Nov 2004 (doi:10.1182/blood-2004-06-2334).
Xu, Y., Banerjee, D., Huelsken, J., Birchmeier, W. & Sen, J. M. Deletion of β-catenin impairs T cell development. Nature Immunol. 4, 1177–1182 (2003).
Cobas, M. et al. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221–229 (2004). References 48 and 49 describe the phenotype of mice that are deficient in β-catenin; however, they report opposite results.
Staal, F. J., Burgering, B. M., van de Wetering, M. & Clevers, H. C. Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int. Immunol. 11, 317–323 (1999).
Gounari, F. et al. Somatic activation of β-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nature Immunol. 2, 863–869 (2001).
Mulroy, T., Xu, Y. & Sen, J. M. β-Catenin expression enhances generation of mature thymocytes. Int. Immunol. 15, 1485–1494 (2003).
van Ewijk, W., Shores, E. W. & Singer, A. Crosstalk in the mouse thymus. Immunol. Today 15, 214–217 (1994).
Balciunaite, G. et al. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nature Immunol. 3, 1102–1108 (2002).
Willert, J., Epping, M., Pollack, J. R., Brown, P. O. & Nusse, R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. [online] 2, 8 (2002).
van de Wetering, M. et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Staal, F. J. et al. Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J. Immunol. 172, 1099–1108 (2004). This report identifies target genes of the WNT–TCF1 signalling pathway in DN thymocytes. It is the first study that has used DNA microarrays to examine the target genes of a transcription factor that is required for lymphoid development.
Bruhn, L., Munnerlyn, A. & Grosschedl, R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev. 11, 640–653 (1997).
Reya, T. et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13, 15–24 (2000). This paper describes the defect in B-cell development of LEF1-deficient mice. In addition, it shows the importance of WNT signalling for the proliferation of pro-B cells.
Christian, S. L., Sims, P. V. & Gold, M. R. The B cell antigen receptor regulates the transcriptional activator β-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J. Immunol. 169, 758–769 (2002).
Muller-Tidow, C. et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol. Cell. Biol. 24, 2890–2904 (2004).
Lu, D. et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA 101, 3118–3123 (2004).
McWhirter, J. R. et al. Oncogenic homeodomain transcription factor E2A–Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc. Natl Acad. Sci. USA 96, 11464–11469 (1999).
Jamieson, C. H. et al. Granulocyte–macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004). This is the first description of the involvement of dysregulated WNT signalling in a haematological malignancy of humans.
Tian, E. et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349, 2483–2494 (2003).
Derksen, P. W. et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc. Natl Acad. Sci. USA 101, 6122–6127 (2004).
Liang, H. et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4, 349–360 (2003).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature Genet. 33, 416–421 (2003).
Galceran, J., Sustmann, C., Hsu, S. C., Folberth, S. & Grosschedl, R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 18, 2718–2723 (2004).
Acknowledgements
We thank C. E. van der Linden for careful reading of the manuscript. F.J.T.S. is supported by grants from NWO (The Netherlands), the Fifth and Sixth Framework Programmes (European Union) and the Bekales Foundation (Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- PRO-B CELLS
-
Cells at the earliest stage of B-cell development in the bone marrow. They are characterized by incomplete immunoglobulin heavy-chain rearrangement and are defined as CD19+ cytoplasmic IgM− or, sometimes, as B220+ CD43+ (by the Hardy classification scheme).
- PRE-B CELLS
-
(Precursor B cells). Cells at a stage of B-cell development in the bone marrow that are characterized by complete immunoglobulin heavy-chain rearrangement in the absence of immunoglobulin light-chain rearrangement. They express the pre-B-cell receptor, and are phenotypically CD19+ cytoplasmic IgM+ or are sometimes defined as B220+CD43− cell-surface IgM− (by the Hardy classification).
- DOUBLE-NEGATIVE SUBSETS
-
(DN subsets). The most immature thymocytes lack expression of the co-receptors CD4 and CD8, and are referred to as DN cells. This compartment can be further subdivided on the basis of CD25 and CD44 expression into four subpopulations: DN1 (CD25−CD44+), DN2 (CD25+CD44+), DN3 (CD25+CD44−) and DN4 (CD25−CD44−).
- IMMATURE SINGLE-POSITIVE POPULATION
-
(ISP population). Cells at a stage of thymocyte differentiation that occurs between CD4−CD8− double-negative cells and CD4+CD8+ double-positive cells. They are characterized by rapid proliferation and, phenotypically, by the presence of either CD8 (mouse) or CD4 (human), with low levels of CD3 expression (in contrast to the more mature single-positive cells, which are CD3hi).
- PLANAR-CELL POLARITY
-
A morphogenic process in Drosophila melanogaster that orients parallel arrays so that the plane of the epithelium is perpendicular to the apical–basal axis. A homologous process occurs in vertebrates during gastrulation and is sometimes called convergent extension. WNT signalling through JNKs (JUN amino-terminal kinases) is thought to transduce the planar-cell polarity signal.
- ADHERENS JUNCTIONS
-
Specialized intercellular junctions of the plasma membrane, in which the cadherin molecules at the surface of adjacent cells interact in a Ca2+-dependent manner. Actin filaments are linked to these cadherin structures through catenins that are located underneath the junctions.
- PROTEASOMAL DEGRADATION
-
Most of the degradation of cytosolic and nuclear proteins in eukaryotic cells is catalysed by multisubunit proteases known as proteasomes. Targeting of proteins to proteasomes most often occurs through the attachment of multiple ubiquitin tags.
- HIGH-MOBILITY-GROUP BOX
-
(HMG box). A domain present in members of a large protein family that are small non-histone components of chromatin and function in higher-order chromatin structure.
- SWI–SNF
-
An ATP-dependent chromatin-remodelling protein complex that was identified in yeast. Related complexes exist in mammals and are involved in the remodelling of the chromatin of various genes.
- MESODERM
-
In animals with three tissue layers, the mesoderm is the middle layer of tissue, between the ectoderm and the endoderm. In vertebrates, the mesoderm forms the skeleton, muscles, heart, spleen and many other internal organs.
- CONDITIONED MEDIUM
-
Medium collected from culture of a cell line that has been allowed to 'build up' with time so that secreted factors in the medium reach high concentrations. It can be added to cultures of transfected or non-transfected cells, often to provide growth factors and/or cytokines.
- IN VIVO COMPLEMENTATION
-
A method to transfer cells, chromosomes or genes to an organism with a mutant phenotype when the aim is to correct the phenotype. It helps to define a genetic hierarchy between genes and provides an in vivo functional read-out.
- NORTHERN BLOTTING
-
A method for transferring RNA from a denaturing agarose gel to a nitrocellulose filter, on which a particular mRNA can be detected by a suitable DNA probe.
- FETAL THYMIC ORGAN CULTURES
-
(FTOCs). Removal of fetal thymi between embryonic day 14 and 16 allows the analysis of several key processes in thymic development — including antigen-driven positive- and negative-selection events — using in vitro culture. The thymic lobes can also be used to allow the development of progenitor cells that are added to the cultures.
- CRE–LOXP
-
The Cre (cyclization recombinase) protein from bacteriophage P1 excises DNA that is flanked by recombination sequences called loxP sites. These sequences can be introduced at either end of a gene by homologous recombination. Animals carrying LoxP-flanked genes can be made transgenic for the Cre gene, which can be placed under a tissue-specific promoter (for example, Lck for T cells) or an inducible promoter (such as Mx, myxovirus resistance, which is induced by interferon or double-stranded RNA). In the cells that express Cre, the loxP sites are recognized, and the DNA between them is excised, leading to tissue-specific or inducible deletion of the gene of interest.
- TRANSLOCATION
-
Transfer of a segment of a chromosome to a different chromosome, often leading to an abnormal fusion gene or overexpression of one of the genes adjacent to the breakpoint.
- BCR–ABL
-
A tyrosine kinase oncogene. The Abelson leukaemia-virus protein (ABL) is fused with the breakpoint-cluster region (BCR) in the Philadelphia-chromosome translocation found in chronic myeloid leukaemia.
- HEMIZYGOUS
-
A genotype characterized by the presence of both a wild-type and a non-functional allele.
Rights and permissions
About this article
Cite this article
Staal, F., Clevers, H. WNT signalling and haematopoiesis: a WNT–WNT situation. Nat Rev Immunol 5, 21–30 (2005). https://doi.org/10.1038/nri1529
Issue Date:
DOI: https://doi.org/10.1038/nri1529
This article is cited by
-
Emerging strategies for treating autoimmune disease with genetically modified dendritic cells
Cell Communication and Signaling (2024)
-
Hypoxia-induced lncRNA RBM5-AS1 promotes tumorigenesis via activating Wnt/β-catenin signaling in breast cancer
Cell Death & Disease (2022)
-
Single-cell analysis at the protein level delineates intracellular signaling dynamic during hematopoiesis
BMC Biology (2021)
-
Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities
Molecular Cancer (2020)
-
High expression of chaperonin-containing TCP1 subunit 3 may induce dismal prognosis in multiple myeloma
The Pharmacogenomics Journal (2020)