Abstract
The generation of lymphoid tissues during embryogenesis relies on group 3 innate lymphoid cells (ILC3) displaying lymphoid tissue inducer (LTi) activity and expressing the master transcription factor RORγt. Accordingly, RORγt-deficient mice lack ILC3 and lymphoid structures, including lymph nodes (LN). Whereas T-bet affects differentiation and functions of ILC3 postnatally, the role of T-bet in regulating fetal ILC3 and LN formation remains completely unknown. Using multiple mouse models and single-cell analyses of fetal ILCs and ILC progenitors (ILCP), here we identify a key role for T-bet during embryogenesis and show that its deficiency rescues LN formation in RORγt-deficient mice. Mechanistically, T-bet deletion skews the differentiation fate of fetal ILCs and promotes the accumulation of PLZFhi ILCP expressing central LTi molecules in a RORα-dependent fashion. Our data unveil an unexpected role for T-bet and RORα during embryonic ILC function and highlight that RORγt is crucial in counteracting the suppressive effects of T-bet.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Raw transcriptome data reported in this paper are deposited and available from the Gene Expression Omnibus under accession codes GSE161441 (scRNA-seq) and GSE161439 (bulk RNA-seq). Sequencing data were aligned to the mm10 reference transcriptome. All other data supporting the findings of this study are available within the paper or from the corresponding author upon request. Source data are provided with this paper.
References
Harly, C., Cam, M., Kaye, J. & Bhandoola, A. Development and differentiation of early innate lymphoid progenitors. J. Exp. Med. 215, 249–262 (2018).
Mebius, R. E., Streeter, P. R., Michie, S., Butcher, E. C. & Weissman, I. L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3− cells to colonize lymph nodes. Proc. Natl Acad. Sci. USA 93, 11019–11024 (1996).
Veiga-Fernandes, H. et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 446, 547–551 (2007).
Mebius, R. E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 3, 292–303 (2003).
Eberl, G. From induced to programmed lymphoid tissues: the long road to preempt pathogens. Trends Immunol. 28, 423–428 (2007).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Kim, D. et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J. Exp. Med. 192, 1467–1478 (2000).
Onder, L. et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity 47, 80–92.e4 (2017).
Förster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Ansel, K. M. et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Finke, D., Acha-Orbea, H., Mattis, A., Lipp, M. & Kraehenbuhl, J. P. CD4+CD3− cells induce Peyer’s patch development: role of α4β1 integrin activation by CXCR5. Immunity 17, 363–373 (2002).
Sun, Z. et al. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373 (2000).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Klose, C. S. N. et al. A T-bet gradient controls the fate and function of CCR6−RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Rankin, L. C. et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395 (2013).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Sanos, S. L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Zhong, C. et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat. Immunol. 17, 169–178 (2016).
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
Pokrovskii, M. et al. Characterization of transcriptional regulatory networks that promote and restrict identities and functions of intestinal innate lymphoid cells. Immunity 51, 185–197.e6 (2019).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246.e13 (2016).
Seillet, C. et al. Differential requirement for Nfil3 during NK cell development. J. Immunol. 192, 2667–2676 (2014).
Seillet, C. et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J. Exp. Med. 211, 1733–1740 (2014).
Male, V. et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J. Exp. Med. 211, 635–642 (2014).
Xu, W. et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. 10, 2043–2054 (2015).
Ghaedi, M. et al. Common-lymphoid-progenitor-independent pathways of innate and T lymphocyte development. Cell Rep. 15, 471–480 (2016).
Possot, C. et al. Notch signaling is necessary for adult, but not fetal, development of RORγ+ innate lymphoid cells. Nat. Immunol. 12, 949–958 (2011).
Mielke, L. A. et al. TCF-1 controls ILC2 and NKp46+ RORγt+ innate lymphocyte differentiation and protection in intestinal inflammation. J. Immunol. 191, 4383–4391 (2013).
Yang, Q. et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity 38, 694–704 (2013).
Seillet, C. et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 17, 436–447 (2016).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Yu, Y. et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 539, 102–106 (2016).
Chea, S. et al. Single-cell gene expression analyses reveal heterogeneous responsiveness of fetal innate lymphoid progenitors to notch signaling. Cell Rep. 14, 1500–1516 (2016).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Hepworth, M. R. et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
von Burg, N. et al. Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc. Natl Acad. Sci. USA 111, 12835–12840 (2014).
Mackley, E. C. et al. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 6, 5862 (2015).
Lehmann, F. M. et al. Microbiota-induced tissue signals regulate ILC3-mediated antigen presentation. Nat. Commun. 11, 1794 (2020).
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751 (2010).
Bernink, J. H. et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 14, 221–229 (2013).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of Cd127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Hanash, A. M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Robinette, M. L. et al. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 8, 14601 (2017).
He, Y.-W. et al. Down-regulation of the orphan nuclear receptor RORγt is essential for T lymphocyte maturation. J. Immunol. 164, 5668–5674 (2000).
Guo, Y. et al. Inhibition of RORγT skews TCRα gene rearrangement and limits T cell repertoire diversity. Cell Rep. 17, 3206–3218 (2016).
Castro, G. et al. RORγt and RORα signature genes in human Th17 cells. PLoS ONE 12, e0181868 (2017).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Sundrud, M. S. & Rao, A. Regulation of T helper 17 differentiation by orphan nuclear receptors: it’s not just RORγt anymore. Immunity 28, 5–7 (2008).
Hernández, P. P. et al. Single-cell transcriptional analysis reveals ILC-like cells in zebrafish. Sci. Immunol. 3, 5265 (2018).
Bando, J. K., Liang, H. E. & Locksley, R. M. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat. Immunol. 16, 153–160 (2015).
Simic, M. et al. Distinct waves from the hemogenic endothelium give rise to layered lymphoid tissue inducer cell ontogeny. Cell Rep. 32, 108004 (2020).
Xu, W. et al. An Id2RFP-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity 50, 1054–1068.e3 (2019).
Walker, J. A. et al. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity 51, 104–118.e7 (2019).
Ishizuka, I. E. et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat. Immunol. 17, 269–276 (2016).
Fiancette, R. et al. Reciprocal transcription factor networks govern tissue-resident ILC3 subset function and identity. Nat. Immunol. https://doi.org/10.1038/s41590-021-01024-x (2021).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673 (2012).
Finotto, S. et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295, 336–338 (2002).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Eberl, G. & Litman, D. R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Haddad, R. et al. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci. 16, 949–957 (2013).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Schlenner, S. M. et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436 (2010).
Paclik, D., Stehle, C., Lahmann, A., Hutloff, A. & Romagnani, C. ICOS regulates the pool of group 2 innate lymphoid cells under homeostatic and inflammatory conditions in mice. Eur. J. Immunol. 45, 2766–2772 (2015).
Lee, Y. et al. Recruitment and activation of naive T cells in the islets by lymphotoxin β receptor-dependent tertiary lymphoid structure. Immunity 25, 499–509 (2006).
Cossarizza, A. et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 47, 1584–1797 (2017).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Roelli, P., bbimber, Flynn, B., santiagorevale & Gui, G. Hoohm/CITE-seq-Count v.1.4.2. https://doi.org/10.5281/ZENODO.2590196 (Zenodo, 2019).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Lun, A. T. L., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with BioConductor. F1000 Res. 5, 2122 (2016).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Wolf, F. A. et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59 (2019).
Philipp, A., Eraslan, G., Virshup, I. & Gigante, S. theislab/anndata2ri v.1.0.4. https://doi.org/10.5281/ZENODO.3992373 (Zenodo, 2020).
Acknowledgements
We thank J. Kirsch, T. Kaiser (Flow Cytometry Core Facility, Deutsches Rheuma-Forschungszentrum, Berlin) and K. Lehmann (Deutsches Rheuma-Forschungszentrum) for technical help. We thank M. Babic for precise help with detection of LN phenotypes; H.-R. Rodewald (Division for Cellular Immunology, German Cancer Research Center, Heidelberg, Germany) and J. Zhu (Molecular and Cellular Immunoregulation Unit, Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA) for providing Il7rcre and Tbx21-ZsGreen mice, respectively; A. Diefenbach and M. Hepworth for scientific discussion; G. Gasteiger, C. Klose, T. Schüler, D. Hernández, J. Zhu and A. Kruglov for critically reading the paper. Funding came from Deutsche Forschungsgemeinschaft (DFG) grants from the Heisenberg Program nos. (RO3565/1-1), RO3565/2-1, SPP 1937 (RO3565/4-1 and 4-2) to C.R., DFG-SFB TRR241 B02 to C.R and B03 to H.D.C., R.M. Schwiete Foundation to H.D.C., Leibniz-Science Campus Chronic Inflammation and Leibniz-Kooperative Exzellenz K259/2019 to C.R. and INST 335/597-1 FUGG. SPP1937 (HA5354/8-1 and 8-2) and TRR130, TP17 to A.E.H, and the state of Berlin and the ‘European Regional Development Fund’ (ERDF 2014–2020, EFRE 1.8/11, Deutsches Rheuma-Forschungszentrum) to M-F.M.)
Author information
Authors and Affiliations
Contributions
C.S. designed, performed and analyzed experiments and wrote the paper. T.R. designed, performed and analyzed single-cell experiment together with C.S. R.F., D.W.G. and C.W. performed experiments. P.D. and M-F.M. performed and analyzed the bulk sequencing experiment together with C.S. and T.R. C.U. and A.E.H performed immunofluorescence experiments. D.F. and D.R.W. provided mouse mutants. H-D.C. designed experiments. J.Z. generated DKO mice, discovered the initial phenotype, designed experiments and edited the manuscript. C.R. supervised the study and wrote the paper together with C.S., with input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Laurie Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single-cell sequencing of E18.5 intestinal cells identifies ILC progenitors and mature subsets.
a, Flow cytometric representative plots showing expression of transcription factors on CD127+ and/or CD122+ on LinLD−CD45+ cells of the E18.5 small intestine (SI). (b) Violin plots of selected dendritic cell (DC)-associated markers. (c) Heatmap displaying the top 50 differentially expressed genes (DEGs) within each cluster. Cluster gene examples are given. (d) UMAP-projected expression of Eomes transcripts. (e) Trajectory analysis using Partition-based Graph Abstraction (PAGA) lineage interference method.
Extended Data Fig. 2 Gene expression of specified ILC3 subsets defined by scRNA-seq.
a, Heatmap displaying the top 30 differentially expressed genes (DEGs) and (b) expression levels of selected genes and corresponding proteins as assessed by CITE-seq within ILC3 subclusters.
Extended Data Fig. 3 Gene expression of specified ILC3 subsets defined by scRNA-seq.
a, Heatmap displaying the top 30 differentially expressed genes (DEGs) and (b) expression levels of selected genes and corresponding proteins as assessed by CITE-seq within ILC3 subclusters.
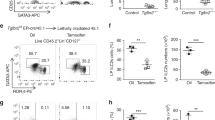
Extended Data Fig. 4 T-bet-deficient mice have normal LN development.
a, In vivo photos of mesenteric or inguinal lymph nodes (mLN, iLN) of adult Tbx21−/− x Rorc(gt)GFP/GFP mice or after isolation (b). (c) Quantification (n = 12) of frequencies of mice with lymphoid structures compared to control mouse strain in adult animals. PP’s, Peyer’s patches; nd, not detected. Data are representative of 2 independent experiments. (d) Photos of isolated mLN in indicated mouse strains. Data are representative of 2 independent experiments.
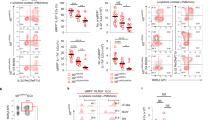
Extended Data Fig. 5 Comparative gene expression of ILCP from reporter, RKO and DKO mice.
a, Violin plots for enrichment score of gene modules of top 100 differentially expressed genes from reporter ILC2 within ILC2 cluster in indicated mouse strains. Statistical significance was calculated using two-sided Wilcoxon test with Bonferroni correction, all ns. (b) Eomes expression in DKO mice projected on UMAP. (c) Expression of selected ILCP-associated genes in cells from ILCP cluster in indicated mouse strains. (d) Expression of selected proteins on UMAP projection analysed by CITE-Seq. (e) Violin plot for Mki67 transcripts among ILCP in all mouse strains, legend see in (c). (f) Representative flow cytometry of Ki67 expression in LinLD−CD45+ CD127+ RORγt−PD-1hiPLZFhi cells from E15.5 SI (reporter n = 6, RKO n = 4, DKO n = 6 from 2 independent experiments). Quantification as mean ± SEM with Kruskal–Wallis significance and Dunn’s correction. Detailed statistics are available in source data.
Extended Data Fig. 6 Gene and protein expression patterns in ILCP of reporter, RKO and DKO mouse strains.
a, Expression levels of Tbx21 within ILCP of all strains. (b) Gene expression profiles of the top 100 genes differentially expressed in ILC3 of reporter mice within cells from ILCP cluster in the different mouse strains. (c) Violin plots depicting expression of selected transcripts in ILCP of the three mouse strains, legend see in (A). (d) Violin plot depicting Rorc expression within ILCP of indicated mouse strains. (e) Geometric mean fluorescence intensity of CXCR5 and RANKL within LinLD−CD45+ CD127+ and/or CD122+ cells isolated from E18.5 SI (n = 7 from 2 independent experiments) depicted as mean ± SEM and Kruskal-Wallis significance with Dunn’s correction. Data from two independent experiments. (f) Representative flow cytometry from E18.5 SI of LinLD−CD45+ CD127+ and/or CD122+ cells and quantification as mean ± SEM of GFP+ cells (n = 7 from 2 independent experiments) with Kruskal-Wallis significance and Dunn’s correction. (g) Frequencies of CXCR5+ RANKL+ cells among LinLD−CD45+ CD127+ and/or CD122+ cells in fetal liver (FL) of E18.5 embryos (n = 4 from 2 independent experiments) with Kruskal-Wallis significance and Dunn’s correction. (h) In vitro differentiation of E14.5 fetal liver-derived ILC progenitors on OP9 stromal cells for 5–8 days in the presence of SCF and IL-7 and analysis by flow cytometry. Quantification of LinLD−CD45+ ICOShiGATA3hi ILC2 and LinLD−CD45+ ICOS− NK1.1+ group 1 ILCs cells in the different mouse strains shown as mean ± SEM. Data are representative of 2-3 independent experiments (reporter n = 5 from 2 independent experiments, RKO n = 9 and DKO n = 11 from 3 independent experiments). Kruskal-Wallis significance and Dunn’s correction. Detailed statistics are available in source data.
Extended Data Fig. 7 GFP+ ILCs persist in double knockout mouse models and develop independently from RAG proteins.
a, Flow cytometry representative plots of mLN from 4-week-old mice of indicated mouse strains. Quantification of frequencies from two independent experiments depicted as mean ± SEM (reporter n = 14, DKO n = 8 from 4 independent experiments). Kruskal-Wallis testing with Dunn’s multiple comparison correction. (b) Representative flow cytometry of cells from SI isolated from 4-week-old mice. Quantification of frequencies from 2 independent experiments as mean ± SEM, Kruskal-Wallis testing with Dunn’s multiple correction, n = 4. P values are provided in source data. Detailed statistics are available in source data.
Extended Data Fig. 8 Thymic CD4+ CD8+ compartments are not restored in RORγt/T-bet double-deficient mice.
a, Mapping of reads detected by bulk RNA-seq to the mm10 mouse Rorc locus in indicated mouse strains. Red box designates localization of transcriptional start site (TSS) ATG of exon 1γt. (b) Expression of Rorc exon1-3 and Rorc exon5-6 junctions in small intestinal LinLD−CD45+ CD3− GFP+ ILCs of 4-week-old mice determined by quantitative PCR. Values are normalized to housekeeping gene Gapdh. Each symbol represents an individual mouse. Data show mean ± SEM, reporter and DKO n = 6, RKO n = 2 examined over 2 independent experiments. (c,d,e) Representative flow cytometry plots of indicated populations and compartments isolated from 4-week-old mice. (f) Violin plot of Rora expression within ILCP of designated mouse strains from E18.5 scRNA-seq dataset. Detailed statistics are available in source data.
Supplementary information
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Stehle, C., Rückert, T., Fiancette, R. et al. T-bet and RORα control lymph node formation by regulating embryonic innate lymphoid cell differentiation. Nat Immunol 22, 1231–1244 (2021). https://doi.org/10.1038/s41590-021-01029-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-01029-6
This article is cited by
-
Cxxc finger protein 1 maintains homeostasis and function of intestinal group 3 innate lymphoid cells with aging
Nature Aging (2023)
-
Transcriptional control of mature ILC3 function and plasticity: not just RORγt
Cellular & Molecular Immunology (2022)
-
Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota
Nature (2022)
-
Reciprocal transcription factor networks govern tissue-resident ILC3 subset function and identity
Nature Immunology (2021)