Key Points
-
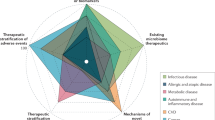
Basic science advances herald an era in which IBD will be subcategorized on the basis of the involvement of specific molecular pathways
-
Knowledge of variation in IBD-susceptibility genes has expanded our understanding of the biological pathways relevant to disease susceptibility and to clinical phenomena including disease location and therapeutic response
-
Multimodal algorithms that combine clinical and genetic information will show utility in diagnostic panels and for predicting disease course and therapeutic response
-
Gene expression signatures and composite models that reflect the influence of environmental factors, such as the microbiome, show promise as tools for individualized risk stratification and treatment selection
Abstract
The expanding knowledge of the role of genetic variants involved in the susceptibility to IBD heralds an era of disease categorization beyond Crohn's disease and ulcerative colitis. A more robust molecular definition of the spectrum of IBD subtypes is likely to be based on specific molecular pathways that determine not only disease susceptibility but also disease characteristics such as location, natural history and therapeutic response. Evolving diagnostic panels for IBD will include clinical variables and genetic markers as well as other indicators of gene function and interaction with environmental factors, such as the microbiome. Multimodal algorithms that combine clinical, serologic and genetic information are likely to be useful in predicting disease course. Variation in IBD-susceptibility and drug-related pathway genes seems to influence the response to anti-TNF therapy. Furthermore, gene expression signatures and composite models have both shown promise as predictors of therapeutic response. Ultimately, models based on combinations of genotype and gene expression data with clinical, biochemical, serological, and microbiome data for clinically meaningful subgroups of patients should permit the development of tools for individualized risk stratification and treatment selection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Vora, P., Shih, D. Q., McGovern, D. P. & Targan, S. R. Current concepts on the immunopathogenesis of inflammatory bowel disease. Front. Biosci. (Elite Ed). 4, 1451–1477 (2012).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Franke, A. et al. Genome-wide association study for ulcerative colitis identifies risk loci at 7q22 and 22q13 (IL17REL). Nat. Genet. 42, 292–294 (2010).
Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43, 246–252 (2011).
Lees, C. W., Barrett, J. C., Parkes, M. & Satsangi, J. New IBD genetics: common pathways with other diseases. Gut 60, 1739–1753 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Ng, P. C., Murray, S. S., Levy, S. & Venter, J. C. An agenda for personalized medicine. Nature 461, 724–726 (2009).
Molodecky, N. A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54.e42 (2012).
McGovern, D. P. et al. TUCAN (CARD8) genetic variants and inflammatory bowel disease. Gastroenterology. 131, 1190–1196 (2006).
Prideaux, L., De Cruz, P., Ng, S. C. & Kamm, M. A. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 18, 1340–1355 (2012).
Zhang, Z. et al. Anti-Saccharomyces cerevisiae antibodies associate with phenotypes and higher risk for surgery in Crohn's disease: a meta-analysis. Dig. Dis. Sci. 57, 2944–2954 (2012).
Kaul, A. et al. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflamm. Bowel Dis. 18, 1872–1884 (2012).
Lakatos, P. L., Papp, M. & Rieder, F. Serologic antiglycan antibodies in inflammatory bowel disease. Am. J. Gastroenterol. 106, 406–412 (2011).
Main, J. et al. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ 297, 1105–1106 (1988).
Rump, J. A. et al. A new type of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) in active ulcerative colitis but not in Crohn's disease. Immunobiology. 181, 406–413 (1990).
Duerr, R. H., Targan, S. R., Landers, C. J., Sutherland, L. R. & Shanahan, F. Anti-neutrophil cytoplasmic antibodies in ulcerative colitis. Comparison with other colitides/diarrheal illnesses. Gastroenterology 100, 1590–1596 (1991).
Vasiliauskas, E. A. et al. Perinuclear antineutrophil cytoplasmic antibodies in patients with Crohn's disease define a clinical subgroup. Gastroenterology 110, 1810–1819 (1996).
Cohavy, O. et al. Colonic bacteria express an ulcerative colitis pANCA-related protein epitope. Infect. Immun. 68, 1542–1548 (2000).
Sutton, C. L. et al. Identification of a novel bacterial sequence associated with Crohn's disease. Gastroenterology 119, 23–31 (2000).
Lodes, M. J. et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004).
Dotan, I. et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology 131, 366–378 (2006).
Quinton, J. F. et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut 42, 788–791 (1998).
Panaccione, R. & Sandborn, W. J. Is antibody testing for inflammatory bowel disease clinically useful? Gastroenterology 116, 1001–1002 (1999).
Zholudev, A., Zurakowski, D., Young, W., Leichtner, A. & Bousvaros, A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn's disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am. J. Gastroenterol. 99, 2235–2241 (2004).
Joossens, S. et al. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut 55, 1667–1669 (2006).
Plevy, S. et al. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn's disease, and ulcerative colitis patients. Inflamm. Bowel Dis. 19, 1139–1148 (2013).
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Jarnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124, 1767–1773 (2003).
Willing, B. P. et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 139, 1844–1854.e1 (2010).
Hansen, J., Gulati, A. & Sartor, R. B. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr. Opin. Gastroenterol. 26, 564–571 (2010).
Manichanh, C. et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211 (2006).
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Qin, J. et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 (2010).
Joossens, M. et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut 60, 631–637 (2011).
Lepage, P. et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 141, 227–236 (2011).
Louis, E. et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut 49, 777–782 (2001).
Satsangi, J. et al. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet 347, 1212–1217 (1996).
Roussomoustakaki, M. et al. Genetic markers may predict disease behavior in patients with ulcerative colitis. Gastroenterology 112, 1845–1853 (1997).
Silverberg, M. S. et al. A population- and family-based study of Canadian families reveals association of HLA DRB1*0103 with colonic involvement in inflammatory bowel disease. Inflamm. Bowel Dis. 9, 1–9 (2003).
Ahmad, T. et al. The contribution of human leucocyte antigen complex genes to disease phenotype in ulcerative colitis. Tissue Antigens 62, 527–535 (2003).
Fernandez, L. et al. IBD1 and IBD3 determine location of Crohn's disease in the Spanish population. Inflamm. Bowel Dis. 10, 715–722 (2004).
Newman, B. et al. CARD15 and HLA DRB1 alleles influence susceptibility and disease localization in Crohn's disease. Am. J. Gastroenterol. 99, 306–315 (2004).
Fisher, S. A. et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn's disease. Nat. Genet. 40, 710–712 (2008).
Loftus, E. V. Jr et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54, 91–96 (2005).
Cho, J. H. & Brant, S. R. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology 140, 1704–1712 (2011).
Janse, M. et al. Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL, and CARD9. Hepatology 53, 1977–1985 (2011).
Melum, E. et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat. Genet. 43, 17–19 (2011).
Wells, C. Ulcerative colitis and Crohn's disease. Ann. R. Coll. Surg. Engl. 11, 105–120 (1952).
Crohn, B. B., Ginzburg, L. & Oppenheimer, G. D. Regional ileitis: a pathologic and clinical entity. JAMA 99, 1323–1329 (1932).
Lesage, S. et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am. J. Hum. Genet. 70, 845–857 (2002).
Brant, S. R. et al. Defining complex contributions of NOD2/CARD15 gene mutations, age at onset, and tobacco use on Crohn's disease phenotypes. Inflamm. Bowel Dis. 9, 281–289 (2003).
Radford-Smith, G. & Pandeya, N. Associations between NOD2/CARD15 genotype and phenotype in Crohn's disease—are we there yet? World J. Gastroenterol. 12, 7097–7103 (2006).
Economou, M., Trikalinos, T. A., Loizou, K. T., Tsianos, E. V. & Ioannidis, J. P. Differential effects of NOD2 variants on Crohn's disease risk and phenotype in diverse populations: a metaanalysis. Am. J. Gastroenterol. 99, 2393–2404 (2004).
Prescott, N. J. et al. A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5. Gastroenterology 132, 1665–1671 (2007).
Roberts, R. L. et al. IL23R R381Q and ATG16L1 T300A are strongly associated with Crohn's disease in a study of New Zealand Caucasians with inflammatory bowel disease. Am. J. Gastroenterol. 102, 2754–2761 (2007).
Latiano, A. et al. Polymorphism of the IRGM gene might predispose to fistulizing behavior in Crohn's disease. Am. J. Gastroenterol. 104, 110–116 (2009).
Weersma, R. K. et al. ATG16L1 and IL23R are associated with inflammatory bowel diseases but not with celiac disease in the Netherlands. Am. J. Gastroenterol. 103, 621–627 (2008).
Tremelling, M. et al. IL23R variation determines susceptibility but not disease phenotype in inflammatory bowel disease. Gastroenterology 132, 1657–1664 (2007).
Jung, C. et al. Genotype/phenotype analyses for 53 Crohn's disease associated genetic polymorphisms. PLoS ONE 7, e52223 (2012).
Cleynen, I. et al. Molecular reclassification of Crohn's disease by cluster analysis of genetic variants. PLoS ONE 5, e12952 (2010).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N. Engl. J. Med. 362, 1383–1395 (2010).
D'Haens, G. et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 371, 660–667 (2008).
Peyrin-Biroulet, L., Bigard, M. A., Malesci, A. & Danese, S. Step-up and top-down approaches to the treatment of Crohn's disease: early may already be too late. Gastroenterology 135, 1420–1422 (2008).
Peyrin-Biroulet, L., Loftus, E. V. Jr, Colombel, J. F. & Sandborn, W. J. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 59, 141–147 (2010).
Pariente, B. et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm. Bowel Dis. 17, 1415–1422 (2011).
Peyrin-Biroulet, L. et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 61, 241–247 (2012).
Beaugerie, L., Seksik, P., Nion-Larmurier, I., Gendre, J. P. & Cosnes, J. Predictors of Crohn's disease. Gastroenterology 130, 650–656 (2006).
Rutgeerts, P. et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 25, 665–672 (1984).
Rutgeerts, P. et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 99, 956–963 (1990).
Baert, F. et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology 138, 463–468 (2010).
Froslie, K. F., Jahnsen, J., Moum, B. A. & Vatn, M. H. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133, 412–422 (2007).
Pineton de Chambrun, G., Peyrin-Biroulet, L., Lemann, M. & Colombel, J. F. Clinical implications of mucosal healing for the management of IBD. Nat. Rev. Gastroenterol. Hepatol. 7, 15–29 (2010).
Mekhjian, H. S., Switz, D. M., Melnyk, C. S., Rankin, G. B. & Brooks, R. K. Clinical features and natural history of Crohn's disease. Gastroenterology 77, 898–906 (1979).
Bernell, O., Lapidus, A. & Hellers, G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann. Surg. 231, 38–45 (2000).
Allez, M. et al. Long term outcome of patients with active Crohn's disease exhibiting extensive and deep ulcerations at colonoscopy. Am. J. Gastroenterol. 97, 947–953 (2002).
Wolters, F. L. et al. Phenotype at diagnosis predicts recurrence rates in Crohn's disease. Gut 55, 1124–1130 (2006).
Solberg, I. C. et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin. Gastroenterol. Hepatol. 5, 1430–1438 (2007).
Loly, C., Belaiche, J. & Louis, E. Predictors of severe Crohn's disease. Scand. J. Gastroenterol. 43, 948–954 (2008).
Tarrant, K. M., Barclay, M. L., Frampton, C. M. & Gearry, R. B. Perianal disease predicts changes in Crohn's disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am. J. Gastroenterol. 103, 3082–3093 (2008).
Romberg-Camps, M. J. et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am. J. Gastroenterol. 104, 371–383 (2009).
Thia, K. T., Sandborn, W. J., Harmsen, W. S., Zinsmeister, A. R. & Loftus, E. V. Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology 139, 1147–1155 (2010).
Beaugerie, L. et al. Impact of cessation of smoking on the course of ulcerative colitis. Am. J. Gastroenterol. 96, 2113–2116 (2001).
Cosnes, J., Carbonnel, F., Beaugerie, L., Le Quintrec, Y. & Gendre, J. P. Effects of cigarette smoking on the long-term course of Crohn's disease. Gastroenterology 110, 424–431 (1996).
Carbonnel, F., Macaigne, G., Beaugerie, L., Gendre, J. P. & Cosnes, J. Crohn's disease severity in familial and sporadic cases. Gut 44, 91–95 (1999).
Farhi, D. et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2,402 patients. Medicine (Baltimore) 87, 281–293 (2008).
Henriksen, M., Jahnsen, J., Lygren, I., Vatn, M. H. & Moum, B. Are there any differences in phenotype or disease course between familial and sporadic cases of inflammatory bowel disease? Results of a population-based follow-up study. Am. J. Gastroenterol. 102, 1955–1963 (2007).
Hoie, O. et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am. J. Gastroenterol. 102, 1692–1701 (2007).
Hoie, O. et al. Low colectomy rates in ulcerative colitis in an unselected European cohort followed for 10 years. Gastroenterology 132, 507–515 (2007).
Gower-Rousseau, C. et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am. J. Gastroenterol. 104, 2080–2088 (2009).
Solberg, I. C. et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand. J. Gastroenterol. 44, 431–440 (2009).
Gisbert, J. P., McNicholl, A. G. & Gomollon, F. Questions and answers on the role of fecal lactoferrin as a biological marker in inflammatory bowel disease. Inflamm. Bowel Dis. 15, 1746–1754 (2009).
Lewis, J. D. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 140, 1817–1826 e1812 (2011).
Iskandar, H. N. & Ciorba, M. A. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl. Res. 159, 313–325 (2012).
Henriksen, M. et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 57, 1518–1523 (2008).
Fagan, E. A. et al. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur. J. Clin. Invest. 12, 351–359 (1982).
Solem, C. A. et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 11, 707–712 (2005).
Schoepfer, A. M. et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 105, 162–169 (2010).
Kolho, K. L., Raivio, T., Lindahl, H. & Savilahti, E. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand. J. Gastroenterol. 41, 720–725 (2006).
Sipponen, T. et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm. Bowel Dis. 14, 1392–1398 (2008).
Sipponen, T. et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn's disease treatment. Scand. J. Gastroenterol. 45, 325–331 (2010).
D'Haens, G. et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 2218–2224 (2012).
Tibble, J. A., Sigthorsson, G., Bridger, S., Fagerhol, M. K. & Bjarnason, I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology 119, 15–22 (2000).
Costa, F. et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn's disease. Gut 54, 364–368 (2005).
D'Inca, R. et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am. J. Gastroenterol. 103, 2007–2014 (2008).
Gisbert, J. P. et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm. Bowel Dis. 15, 1190–1198 (2009).
Garcia-Sanchez, V. et al. Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J. Crohns Colitis 4, 144–152 (2010).
Kallel, L. et al. Fecal calprotectin is a predictive marker of relapse in Crohn's disease involving the colon: a prospective study. Eur. J. Gastroenterol. Hepatol. 22, 340–345 (2010).
Laharie, D. et al. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment. Pharmacol. Ther. 34, 462–469 (2011).
Dabritz, J. et al. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm. Bowel Dis. 19, 1130–1138 (2013).
Sandborn, W. J., Landers, C. J., Tremaine, W. J. & Targan, S. R. Association of antineutrophil cytoplasmic antibodies with resistance to treatment of left-sided ulcerative colitis: results of a pilot study. Mayo Clin. Proc. 71, 431–436 (1996).
Fleshner, P. R. et al. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut 49, 671–677 (2001).
Solberg, I. C. Predictive value of serologic markers in a population-based Norwegian cohort with inflammatory bowel disease. Inflamm. Bowel Dis. 15, 406–414 (2009).
Vasiliauskas, E. A. et al. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut 47, 487–496 (2000).
Mow, W. S. et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology 126, 414–424 (2004).
Targan, S. R. et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology 128, 2020–2028 (2005).
Dubinsky, M. C. et al. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. Am. J. Gastroenterology. 101, 360–367 (2006).
Arnott, I. D. et al. Sero-reactivity to microbial components in Crohn's disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am. J. Gastroenterol. 99, 2376–2384 (2004).
Papp, M. et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am. J. Gastroenterol. 103, 665–681 (2008).
Forcione, D. G., Rosen, M. J., Kisiel, J. B. & Sands, B. E. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn's disease. Gut 53, 1117–1122 (2004).
Dendrinos, K. G. et al. Anti-Saccharomyces cerevisiae antibodies are associated with the development of postoperative fistulas following ileal pouch-anal anastomosis. J. Gastrointest. Surg. 10, 1060–1064 (2006).
Melmed, G. Y. et al. Family history and serology predict Crohn's disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis. Colon Rectum 51, 100–108 (2008).
Papadakis, K. A. et al. Anti-flagellin (CBir1) phenotypic and genetic Crohn's disease associations. Inflamm. Bowel Dis. 13, 524–530 (2007).
Ferrante, M. et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut 56, 1394–1403 (2007).
Dubinsky, M. C. et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin. Gastroenterol. Hepatol. 6, 1105–1111 (2008).
Seow, C. H. et al. Novel anti-glycan antibodies related to inflammatory bowel disease diagnosis and phenotype. Am. J. Gastroenterol. 104, 1426–1434 (2009).
Rieder, F. et al. Serum anti-glycan antibodies predict complicated Crohn's disease behavior: a cohort study. Inflamm. Bowel Dis. 16, 1367–1375 (2010).
Vermeire, S. et al. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm. Bowel Dis. 7, 8–15 (2001).
Malickova, K., Lukas, M., Donoval, R., Sandova, P. & Janatkova, I. Novel anti-carbohydrate autoantibodies in patients with inflammatory bowel disease: are they useful for clinical practice? Clin. Lab. 52, 631–638 (2006).
Bossuyt, X. Serologic markers in inflammatory bowel disease. Clin. Chem. 52, 171–181 (2006).
Rieder, F. et al. Characterization of changes in serum anti-glycan antibodies in Crohn's disease—a longitudinal analysis. PLoS ONE 6, e18172 (2011).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Genetic analysis to predict prognosis at the onset of Crohn's disease: not yet ready for prime time? Gut 58, 323–324 (2009).
Hancock, L. et al. Clinical and molecular characteristics of isolated colonic Crohn's disease. Inflamm. Bowel Dis. 14, 1667–1677 (2008).
Ho, G. T. et al. ABCB1/MDR1 gene determines susceptibility and phenotype in ulcerative colitis: discrimination of critical variants using a gene-wide haplotype tagging approach. Hum. Mol. Genet. 15, 797–805 (2006).
Haritunians, T. et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm. Bowel Dis. 16, 1830–1840 (2010).
Abreu, M. T. et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology 123, 679–688 (2002).
Vermeire, S. et al. CARD15 genetic variation in a Quebec population: prevalence, genotype-phenotype relationship, and haplotype structure. Am. J. Hum. Genet. 71, 74–83 (2002).
Hampe, J. et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet 359, 1661–1665 (2002).
Cuthbert, A. P. et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 122, 867–874 (2002).
Alvarez-Lobos, M. et al. Crohn's disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann. Surg. 242, 693–700 (2005).
Seiderer, J. et al. Predictive value of the CARD15 variant 1007fs for the diagnosis of intestinal stenoses and the need for surgery in Crohn's disease in clinical practice: results of a prospective study. Inflamm. Bowel Dis. 12, 1114–1121 (2006).
Nasir, B. F. et al. Perianal disease combined with NOD2 genotype predicts need for IBD-related surgery in Crohn's disease patients from a population-based cohort. J. Clin. Gastroenterol. 47, 242–245 (2013).
Adler, J., Rangwalla, S. C., Dwamena, B. A. & Higgins, P. D. The prognostic power of the NOD2 genotype for complicated Crohn's disease: a meta-analysis. Am. J. Gastroenterol. 106, 699–712 (2011).
Weersma, R. K. et al. Molecular prediction of disease risk and severity in a large Dutch Crohn's disease cohort. Gut 58, 388–395 (2009).
Henckaerts, L. et al. Genetic risk profiling and prediction of disease course in Crohn's disease patients. Clin. Gastroenterol. Hepatol. 7, 972–980.e2 (2009).
Cleynen, I. et al. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut 62, 1556–1565 (2012).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Classification of inflammatory bowel disease: the old and the new. Curr. Opin. Gastroenterol. 28, 321–326 (2012).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Michail, S. et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 18, 1799–1808 (2012).
Iliev, I. D. et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336, 1314–1317 (2012).
Lee, J. C. et al. Gene expression profiling of CD8+ T cells predicts prognosis in patients with Crohn disease and ulcerative colitis. J. Clin. Invest. 121, 4170–4179 (2011).
Alex, P., Gucek, M. & Li, X. Applications of proteomics in the study of inflammatory bowel diseases: Current status and future directions with available technologies. Inflamm. Bowel Dis. 15, 616–629 (2009).
Roda, G. et al. New proteomic approaches for biomarker discovery in inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1239–1246 (2010).
Meuwis, M. A. et al. Proteomics for prediction and characterization of response to infliximab in Crohn's disease: a pilot study. Clin. Biochem. 41, 960–967 (2008).
Lin, H. M., Helsby, N. A., Rowan, D. D. & Ferguson, L. R. Using metabolomic analysis to understand inflammatory bowel diseases. Inflamm. Bowel Dis. 17, 1021–1029 (2011).
Devlin, S. M. et al. NOD2 variants and antibody response to microbial antigens in Crohn's disease patients and their unaffected relatives. Gastroenterology 132, 576–586 (2007).
Henckaerts, L. et al. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut 56, 1536–1542 (2007).
Lakatos, P. L. et al. Interaction between seroreactivity to microbial antigens and genetics in Crohn's disease: is there a role for defensins? Tissue Antigens 71, 552–559 (2008).
Lakatos, P. L. et al. Pancreatic autoantibodies are associated with reactivity to microbial antibodies, penetrating disease behavior, perianal disease, and extraintestinal manifestations, but not with NOD2/CARD15 or TLR4 genotype in a Hungarian IBD cohort. Inflamm. Bowel Dis. 15, 365–374 (2009).
Murdoch, T. B. et al. Pattern recognition receptor and autophagy gene variants are associated with development of antimicrobial antibodies in Crohn's disease. Inflamm. Bowel Dis. 18, 1743–1748 (2012).
Ippoliti, A. et al. Combination of innate and adaptive immune alterations increased the likelihood of fibrostenosis in Crohn's disease. Inflamm. Bowel Dis. 16, 1279–1285 (2010).
Lichtenstein, G. R. et al. Combination of genetic and quantitative serological immune markers are associated with complicated Crohn's disease behavior. Inflamm. Bowel Dis. 17, 2488–2496 (2011).
Dubinsky, M. C. et al. Multidimensional Prognostic Risk Assessment Identifies Association Between IL12B Variation and Surgery in Crohn's Disease. Inflamm. Bowel Dis. 19, 1662–1670 (2013).
Kohane, I. S., Drazen, J. M. & Campion, E. W. A glimpse of the next 100 years in medicine. N. Engl. J. Med. 367, 2538–2539 (2012).
Lichtenstein, G. R., Abreu, M. T., Cohen, R., Tremaine, W. & American Gastroenterological Association. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 130, 935–939 (2006).
Winter, J. et al. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 20, 593–599 (2004).
Dubinsky, M. C. et al. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am. J. Gastroenterol. 100, 2239–2247 (2005).
Ordas, I., Mould, D. R., Feagan, B. G. & Sandborn, W. J. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 91, 635–646 (2012).
Vande Casteele, N. et al. Early serial trough and antidrug antibody level measurements predict clinical outcome of infliximab and adalimumab treatment. Gut 61, 321 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Afif, W. et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am. J. Gastroenterol. 105, 1133–1139 (2010).
Velayos, F. S., Kahn, J. G., Sandborn, W. J. & Feagan, B. G. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin. Gastroenterol. Hepatol. 11, 654–666 (2013).
Hanauer, S. B. et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 359, 1541–1549 (2002).
Rutgeerts, P. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353, 2462–2476 (2005).
Colombel, J. F. et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 132, 52–65 (2007).
Schreiber, S. et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N. Engl. J. Med. 357, 239–250 (2007).
Schnitzler, F. et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut 58, 492–500 (2009).
Peyrin-Biroulet, L. & Lemann, M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment. Pharmacol. Ther. 33, 870–879 (2011).
Louis, E. et al. A positive response to infliximab in Crohn disease: association with a higher systemic inflammation before treatment but not with −308 TNF gene polymorphism. Scand. J. Gastroenterol. 37, 818–824 (2002).
Parsi, M. A. S. et al. Predictors of response to infliximab in patients with Crohn's disease. Gastroenterology 123, 707–713 (2002).
Vermeire, S. et al. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn's disease. Am. J. Gastroenterol. 97, 2357–2363 (2002).
Arnott, I. D., McNeill, G. & Satsangi, J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn's disease. Aliment. Pharmacol. Ther. 17, 1451–1457 (2003).
Jurgens, M. et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn's disease. Clin. Gastroenterol. Hepatol. 9, 421–427.e1 (2011).
Taylor, K. D. et al. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn's disease. Gastroenterology 120, 1347–1355 (2001).
Esters, N. et al. Serological markers for prediction of response to anti-tumor necrosis factor treatment in Crohn's disease. Am. J. Gastroenterol. 97, 1458–1462 (2002).
Ferrante, M. et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm. Bowel Dis. 13, 123–128 (2007).
Jurgens, M. et al. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am. J. Gastroenterol. 105, 1811–1819 (2010).
Dubinsky, M. C. et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 16, 1357–1366 (2010).
Mascheretti, S. et al. Pharmacogenetic investigation of the TNF/TNF-receptor system in patients with chronic active Crohn's disease treated with infliximab. Pharmacogenomics J. 2, 127–136 (2002).
Pierik, M. et al. Tumour necrosis factor-alpha receptor 1 and 2 polymorphisms in inflammatory bowel disease and their association with response to infliximab. Aliment. Pharmacol. Ther. 20, 303–310 (2004).
Vermeire, S. et al. NOD2/CARD15 does not influence response to infliximab in Crohn's disease. Gastroenterology 123, 106–111 (2002).
Hlavaty, T. et al. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment. Pharmacol. Ther. 22, 613–626 (2005).
Hlavaty, T. et al. Predictive model for the outcome of infliximab therapy in Crohn's disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm. Bowel Dis. 13, 372–379 (2007).
Arijs, I. et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut 58, 1612–1619 (2009).
Arijs, I. et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn's disease. Inflamm. Bowel Dis. 16, 2090–2098 (2010).
Vermeire, S. Towards a novel molecular classification of IBD. Dig. Dis. 30, 425–427 (2012).
Siegel, C. A. et al. Real-time tool to display the predicted disease course and treatment response for children with Crohn's disease. Inflamm. Bowel Dis. 17, 30–38 (2011).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Author information
Authors and Affiliations
Contributions
M. E. Gerich researched data, discussed content, wrote and reviewed/edited the manuscript. D. P. B. McGovern discussed the content and reviewed/edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
D. P. B. McGovern is on the advisory board for UCB and acts as an advisor for 23andMe. M. E. Gerich declares no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Gerich, M., McGovern, D. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol 11, 287–299 (2014). https://doi.org/10.1038/nrgastro.2013.242
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2013.242
This article is cited by
-
Redefining the IBDs using genome-scale molecular phenotyping
Nature Reviews Gastroenterology & Hepatology (2019)
-
Neue medikamentöse Ansätze bei chronisch-entzündlichen Darmerkrankungen
coloproctology (2019)
-
Recent Advances in the Etiopathogenesis of Inflammatory Bowel Disease: The Role of Omics
Molecular Diagnosis & Therapy (2018)
-
Open: Vedolizumab for Ulcerative Colitis: Treatment Outcomes from the VICTORY Consortium
American Journal of Gastroenterology (2018)
-
Outcomes of Fecal Microbiota Transplantation for Clostridium difficile Infection in Patients with Inflammatory Bowel Disease
Digestive Diseases and Sciences (2017)