Abstract
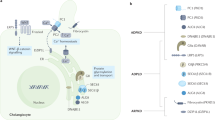
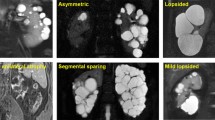
Polycystic liver disease (PLD) is arbitrarily defined as a liver that contains >20 cysts. The condition is associated with two genetically distinct diseases: as a primary phenotype in isolated polycystic liver disease (PCLD) and as an extrarenal manifestation in autosomal dominant polycystic kidney disease (ADPKD). Processes involved in hepatic cystogenesis include ductal plate malformation with concomitant abnormal fluid secretion, altered cell–matrix interaction and cholangiocyte hyperproliferation. PLD is usually a benign disease, but can cause debilitating abdominal symptoms in some patients. The main risk factors for growth of liver cysts are female sex, exogenous oestrogen use and multiple pregnancies. Ultrasonography is very useful for achieving a correct diagnosis of a polycystic liver and to differentiate between ADPKD and PCLD. Current radiological and surgical therapies for symptomatic patients include aspiration–sclerotherapy, fenestration, segmental hepatic resection and liver transplantation. Medical therapies that interact with regulatory mechanisms controlling expansion and growth of liver cysts are under investigation. Somatostatin analogues are promising; several clinical trials have shown that these drugs can reduce the volume of polycystic livers. The purpose of this Review is to provide an update on the diagnosis and management of PLD with a focus on literature published in the past 4 years.
Key Points
-
Polycystic liver disease (PLD) is the primary presentation in isolated polycystic liver disease (PCLD) and can present as an extrarenal manifestation in autosomal dominant polycystic kidney disease
-
Female sex, exogenous oestrogens and multiple pregnancies are risk factors for the development of polycystic livers
-
Ultrasonography is the first step in diagnosing PLD
-
Screening for intracranial aneurysms is not recommended for patients with PCLD
-
Surgical treatment is indicated in symptomatic patients; the choice of treatment depends on total liver volume and the size and location of the liver cysts
-
Somatostatin analogues decrease the volume of polycystic livers, are well-tolerated and improve patients' perception of their health
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Drenth, J. P., Chrispijn, M., Nagorney, D. M., Kamath, P. S. & Torres, V. E. Medical and surgical treatment options for polycystic liver disease. Hepatology 52, 2223–2230 (2010).
van Keimpema, L. et al. Patients with isolated polycystic liver disease referred to liver centres: clinical characterization of 137 cases. Liver Int. 31, 92–98 (2011).
van Gulick, J. J. M., Gevers, T. J., van Keimpema, L. & Drenth, J. P. Hepatic and renal manifestations in autosomal dominant polycystic kidney disease: dichotomy of a spectrum. Neth. J. Med. 69, 367–671 (2011).
Waanders, E. et al. Secondary and tertiary structure modeling reveals effects of novel mutations in polycystic liver disease genes PRKCSH and SEC63. Clin. Genet. 78, 47–56 (2010).
Davila, S. et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 36, 575–577 (2004).
Drenth, J. P., te Morsche, R. H., Smink, R., Bonifacino, J. S. & Jansen, J. B. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat. Genet. 33, 345–347 (2003).
Janssen, M. J., Waanders, E., Woudenberg, J., Lefeber, D. J. & Drenth, J. P. Congenital disorders of glycosylation in hepatology: the example of polycystic liver disease. J. Hepatol. 52, 432–440 (2010).
Everson, G. T., Taylor, M. R. & Doctor, R. B. Polycystic disease of the liver. Hepatology 40, 774–782 (2004).
Torres, V. E. & Harris, P. C. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 (2009).
Torres, V. E., Harris, P. C. & Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 (2007).
Harris, P. C. & Torres, V. E. Polycystic kidney disease. Annu. Rev. Med. 60, 321–337 (2009).
Yoder, B. K. Role of primary cilia in the pathogenesis of polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1381–1388 (2007).
Johnson, A. M. & Gabow, P. A. Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J. Am. Soc. Nephrol. 8, 1560–1567 (1997).
Robinson, C. et al. Clinical utility of PKD2 mutation testing in a polycystic kidney disease cohort attending a specialist nephrology out-patient clinic. BMC Nephrol. 13, 79 (2012).
Drenth, J. P., Martina, J. A., van de Kerkhof, R., Bonifacino, J. S. & Jansen, J. B. Polycystic liver disease is a disorder of cotranslational protein processing. Trends Mol. Med. 11, 37–42 (2005).
Fedeles, S. V. et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 43, 639–647 (2011).
Bergmann, C. & Weiskirchen, R. It's not all in the cilium, but on the road to it: genetic interaction network in polycystic kidney and liver diseases and how trafficking and quality control matter. J. Hepatol. 56, 1201–1203 (2012).
Janssen, M. J. et al. Secondary, somatic mutations might promote cyst formation in patients with autosomal dominant polycystic liver disease. Gastroenterology 141, 2056–2063 e2 (2011).
Banales, J. M., Munoz-Garrido, P. & Bujanda, L. Somatic second-hit mutations leads to polycystic liver diseases. World J. Gastroenterol. (in press).
Strazzabosco, M. & Fabris, L. Development of the bile ducts: essentials for the clinical hepatologist. J. Hepatol. 56, 1159–1170 (2012).
Drenth, J. P., Chrispijn, M. & Bergmann, C. Congenital fibrocystic liver diseases. Best Pract. Res. Clin. Gastroenterol. 24, 573–584 (2010).
Strazzabosco, M. & Somlo, S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology 140, 1855–1859 (2011).
Alvaro, D. et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am. J. Pathol. 172, 321–332 (2008).
Gradilone, S. A. et al. Activation of Trpv4 reduces the hyperproliferative phenotype of cystic cholangiocytes from an animal model of ARPKD. Gastroenterology 139, 304–314 (2010).
Drummond, I. A. Polycystins, focal adhesions and extracellular matrix interactions. Biochim. Biophys. Acta 1812, 1322–1326 (2011).
Banales, J. M. et al. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49, 160–174 (2009).
Spirli, C. et al. Altered store operated calcium entry increases cyclic 3′, 5′-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology 55, 856–868 (2012).
Spirli, C. et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology 56, 2363–2374 (2012).
Masyuk, T. V., Masyuk, A. I., Torres, V. E., Harris, P. C. & LaRusso, N. F. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′, 5′-cyclic monophosphate. Gastroenterology 132, 1104–1116 (2007).
Spirli, C. et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 51, 1778–1788 (2010).
Amura, C. R. et al. VEGF receptor inhibition blocks liver cyst growth in pkd2(WS25/-) mice. Am. J. Physiol. Cell Physiol. 293, C419–C428 (2007).
Qian, Q. et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J. Am. Soc. Nephrol. 19, 631–638 (2008).
Novalic, Z. et al. Dose-dependent effects of sirolimus on mTOR signaling and polycystic kidney disease. J. Am. Soc. Nephrol. 23, 842–853 (2012).
Gevers, T. J. & Drenth, J. P. Somatostatin analogues for treatment of polycystic liver disease. Curr. Opin. Gastroenterol. 27, 294–300 (2011).
Perico, N. et al. Sirolimus therapy to halt the progression of ADPKD. J. Am. Soc. Nephrol. 21, 1031–1040 (2010).
Serra, A. L. et al. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 820–829 (2010).
Walz, G. et al. Everolimus in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 363, 830–840 (2010).
Stallone, G. et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD-study): a randomized, controlled study. Nephrol. Dial. Transplant. 27, 3560–3567 (2012).
Chrispijn, M. & Drenth, J. P. Everolimus and long acting octreotide as a volume reducing treatment of polycystic livers (ELATE): study protocol for a randomized controlled trial. Trials 12, 246 (2011).
Bae, K. T. et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin. J. Am. Soc. Nephrol. 1, 64–69 (2006).
Caroli, A. et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin. J. Am. Soc. Nephrol. 5, 783–789 (2010).
Hogan, M. C. et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J. Am. Soc. Nephrol. 21, 1052–1061 (2010).
van Keimpema, L. et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 137, 1661–1668 (2009).
Hoevenaren, I. A. et al. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 28, 264–270 (2008).
Waanders, E., te Morsche, R. H., de Man, R. A., Jansen, J. B. & Drenth, J. P. Extensive mutational analysis of PRKCSH and SEC63 broadens the spectrum of polycystic liver disease. Hum. Mutat. 27, 830 (2006).
Alvaro, D. et al. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am. J. Pathol. 169, 877–888 (2006).
Chapman, A. B. Cystic disease in women: clinical characteristics and medical management. Adv. Ren. Replace Ther. 10, 24–30 (2003).
Sherstha, R. et al. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 26, 1282–1286 (1997).
Qian, Q. et al. Clinical profile of autosomal dominant polycystic liver disease. Hepatology 37, 164–171 (2003).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Bleeker-Rovers, C. P., Vos, F. J., Corstens, F. H. & Oyen, W. J. Imaging of infectious diseases using [18F] fluorodeoxyglucose PET. Q. J. Nucl. Med. Mol. Imaging 52, 17–29 (2008).
Piccoli, G. B. et al. Positron emission tomography in the diagnostic pathway for intracystic infection in adpkd and “cystic” kidneys. A case series. BMC Nephrol. 12, 48 (2011).
Jouret, F. et al. Positron-emission computed tomography in cyst infection diagnosis in patients with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 1644–1650 (2011).
Fick, G. M. & Gabow, P. A. Natural history of autosomal dominant polycystic kidney disease. Annu. Rev. Med. 45, 23–29 (1994).
Waanders, E. et al. Carbohydrate antigen 19–19 is extremely elevated in polycystic liver disease. Liver Int. 29, 1389–1395 (2009).
Kanaan, N., Goffin, E., Pirson, Y., Devuyst, O. & Hassoun, Z. Carbohydrate antigen 19–19 as a diagnostic marker for hepatic cyst infection in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 55, 916–922 (2010).
Pei, Y. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 20, 205–212 (2009).
Qian, Q. Isolated polycystic liver disease. Adv. Chronic Kidney Dis. 17, 181–189 (2010).
Gigot, J. F. et al. Adult polycystic liver disease: is fenestration the most adequate operation for long-term management? Ann. Surg. 225, 286–294 (1997).
van Keimpema, L., de Koning, D. B., Strijk, S. P. & Drenth, J. P. Aspiration-sclerotherapy results in effective control of liver volume in patients with liver cysts. Dig. Dis. Sci. 53, 2251–2257 (2008).
van Keimpema, L., Ruurda, J. P., Ernst, M. F., van Geffen, H. J. & Drenth, J. P. Laparoscopic fenestration of liver cysts in polycystic liver disease results in a median volume reduction of 12.5%. J. Gastrointest. Surg. 12, 477–482 (2008).
Ecder, T. & Schrier, R. W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 5, 221–228 (2009).
Vlak, M. H., Algra, A., Brandenburg, R. & Rinkel, G. J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 10, 626–636 (2011).
Xu, H. W., Yu, S. Q., Mei, C. L. & Li, M. H. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke 42, 204–206 (2011).
Gevers, T. J., de Koning, D. B., van Dijk, A. P. & Drenth, J. P. Low prevalence of cardiac valve abnormalities in patients with autosomal dominant polycystic liver disease. Liver Int. 32, 690–692 (2012).
Torra, R. et al. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 3, 790–793 (2008).
van Keimpema, L. & Hockerstedt, K. Treatment of polycystic liver disease. Br. J. Surg. 96, 1379–1380 (2009).
Larssen, T. B., Rosendahl, K., Horn, A., Jensen, D. K. & Rorvik, J. Single-session alcohol sclerotherapy in symptomatic benign hepatic cysts performed with a time of exposure to alcohol of 10 min: initial results. Eur. Radiol. 13, 2627–2632 (2003).
Russell, R. T. & Pinson, C. W. Surgical management of polycystic liver disease. World J. Gastroenterol. 13, 5052–5059 (2007).
Martin, I. J., McKinley, A. J., Currie, E. J., Holmes, P. & Garden, O. J. Tailoring the management of nonparasitic liver cysts. Ann. Surg. 228, 167–172 (1998).
Schnelldorfer, T., Torres, V. E., Zakaria, S., Rosen, C. B. & Nagorney, D. M. Polycystic liver disease: a critical appraisal of hepatic resection, cyst fenestration, and liver transplantation. Ann. Surg. 250, 112–118 (2009).
Pirenne, J. et al. Liver transplantation for polycystic liver disease. Liver Transpl. 7, 238–245 (2001).
Coelho-Prabhu, N., Nagorney, D. M. & Baron, T. H. ERCP for the treatment of bile leak after partial hepatectomy and fenestration for symptomatic polycystic liver disease. World J. Gastroenterol. 18, 3705–3709 (2012).
Temmerman, F. et al. Systematic review: the pathophysiology and management of polycystic liver disease. Aliment. Pharmacol. Ther. 34, 702–713 (2011).
Arrazola, L., Moonka, D., Gish, R. G. & Everson, G. T. Model for end-stage liver disease (MELD) exception for polycystic liver disease. Liver Transpl. 12 (Suppl. 3), S110–S111 (2006).
Freeman, R. B. Jr et al. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 12 (Suppl. 3), S128–S136 (2006).
Adam, R. & Hoti, E. Liver transplantation: the current situation. Semin. Liver Dis. 29, 3–18 (2009).
van Keimpema, L. et al. Excellent survival after liver transplantation for isolated polycystic liver disease: an European Liver Transplant Registry study. Transpl. Int. 24, 1239–1245 (2011).
Kirchner, G. I. et al. Outcome and quality of life in patients with polycystic liver disease after liver or combined liver-kidney transplantation. Liver Transpl. 12, 1268–1277 (2006).
Ubara, Y. et al. Intravascular embolization therapy in a patient with an enlarged polycystic liver. Am. J. Kidney Dis. 43, 733–738 (2004).
Bello-Reuss, E., Holubec, K. & Rajaraman, S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 60, 37–45 (2001).
Wang, M. Q., Duan, F., Liu, F. Y., Wang, Z. J. & Song, P. Treatment of symptomatic polycystic liver disease: transcatheter super-selective hepatic arterial embolization using a mixture of NBCA and iodized oil. Abdom. Imaging http://dx.doi.org/10.1007/s00261-012-9931-9931.
Takei, R. et al. Percutaneous transcatheter hepatic artery embolization for liver cysts in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 49, 744–752 (2007).
Patel, Y. C. Somatostatin and its receptor family. Front. Neuroendocrinol. 20, 157–198 (1999).
Temmerman, F. et al. The reduction in liver volume in polycystic liver disease with lanreotide is dose dependent and is most pronounced in patients with the highest liver volume. J. Hepatol. 56, S547 (2012).
Chrispijn, M. et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment. Pharmacol. Ther. 35, 266–274 (2012).
Hogan, M. C. et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol. Dial. Transplant. 27, 3532–3539 (2012).
Modlin, I. M., Pavel, M., Kidd, M. & Gustafsson, B. I. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment. Pharmacol. Ther. 31, 169–188 (2010).
Weckbecker, G., Briner, U., Lewis, I. & Bruns, C. SOM230: a new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs. Endocrinology 143, 4123–4130 (2002).
Lesche, S., Lehmann, D., Nagel, F., Schmid, H. A. & Schulz, S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J. Clin. Endocrinol. Metab. 94, 654–661 (2009).
Acknowledgements
The authors would like to acknowledge the support of the Institute of Genetic and Metabolic Diseases of the Radboud University Nijmegen Medical Centre, The Netherlands. The authors thank the following persons from the Department of Gastroenterology and Hepatology, Radboud University Nijmegen Medical Centre, The Netherlands: Frank Weimer for designing the structure of this paper and drafting the first version, Melissa Chrispijn for expert advice and Marten Lantinga for proofreading the article before submission.
Author information
Authors and Affiliations
Contributions
T. J. G. Gevers researched data for the article. Both authors contributed to discussion of the content and wrote the article. J. P. H. Drenth reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J. P. H. Drenth has received grant support from Ipsen and Novartis.
Rights and permissions
About this article
Cite this article
Gevers, T., Drenth, J. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol 10, 101–108 (2013). https://doi.org/10.1038/nrgastro.2012.254
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2012.254
This article is cited by
-
Polycystic intrahepatic infection caused by Enterococcus casseliflavus: a case report and literature review
BMC Nephrology (2024)
-
Operative Outcomes for Polycystic Liver Disease: Results of a Large Contemporary Series
Journal of Gastrointestinal Surgery (2023)
-
Fatal acute portal vein thrombosis associated with hepatic cysts in a patient with autosomal dominant polycystic kidney disease
CEN Case Reports (2023)
-
Genetics, pathobiology and therapeutic opportunities of polycystic liver disease
Nature Reviews Gastroenterology & Hepatology (2022)
-
Polycystic liver: automatic segmentation using deep learning on CT is faster and as accurate compared to manual segmentation
European Radiology (2022)