Key Points
-
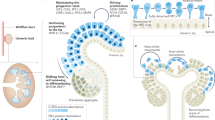
The kidney is an excellent model in which to study the mechanisms of organogenesis.
-
During kidney development, the epithelial ureteric buds branch and induce the surrounding mesenchymal cells to transform into epithelium, which goes on to form the functional unit of the kidney, the nephron.
-
The generation of mouse mutants has lead to the discovery of several genetic cascades that regulate early kidney organogenesis in the mouse.
-
Transcription factors such as Wt1, Pax2, Eya1, Sall1 and Foxc1 contribute to the initiation of organogenesis, and Gdnf signals through the Ret receptor to induce ureteric budding and the regulation of its branching, together with other factors, such as pleiotrophin.
-
Cell-surface proteoglycans, such as glypican-3, and enzymes that regulate the synthesis of the proteoglycans side chains, such as Hs2st, are also important in early kidney development.
-
Wnt signalling is essential for tubulogenesis in the kidney and triggers nephrogenesis in vitro. The bone morphogenetic proteins might regulate the proliferation of nephrogenic cells.
-
The stromal compartment of the kidney is also a significant player in coordinating kidney development. Forkhead box D1 and nuclear retinoic acid receptors are expressed in the stroma and are required for proper kidney development.
-
Gene knockouts in the mouse indicate that signalling from the ureteric bud, kidney mesenchyme and stroma regulate kidney development. However, many of the signals that are involved have yet to be discovered.
Abstract
The kidney is widely used to study the mechanisms of organogenesis. Its development involves fundamental processes, such as epithelial branching, induced morphogenesis and cytodifferentiation, which are common to the development of many other organs. Gene-targeting experiments have greatly improved our understanding of kidney development, and have revealed many important genes that regulate early kidney organogenesis, some of which have a role in inherited human kidney disorders. Although our understanding of how the kidney is assembled is still limited, these studies are beginning to provide insights into the genetic and cellular interactions that regulate early organogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Saxén, L. Organogenesis of the kidney 1–173 (Cambridge Univ. Press, Cambridge, UK, 1987).
Sariola, H. Nephron induction revisited: from caps to condensates. Curr. Opin. Nephrol. Hypertens. 11, 17–21 (2002).
Woolf, A. S. & Loughna, S. Origin of glomerular capillaries: is the verdict in? Exp. Nephrol. 6, 17–21 (1998).
Hammes, A. et al. Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106, 319–329 (2001).
Davies, R. et al. Multiple roles for the Wilms' tumor suppressor, WT1. Cancer Res. 59, 1747s–1750s (1999).
Vilain, E. & McCabe, E. R. Mammalian sex determination: from gonads to brain. Mol. Genet. Metab. 65, 74–84 (1998).
Parker, K. L., Schedl, A. & Schimmer, B. P. Gene interactions in gonadal development. Annu. Rev. Physiol. 61, 417–433 (1999).
Kreidberg, J. A. et al. WT1 is required for early kidney development. Cell 74, 679–691 (1993).
Lee, S. B. et al. The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell 98, 663–673 (1999).
Ryan, G., Steele-Perkins, V., Morris, J. F., Rauscher, F. J. & Dressler, G. R. Repression of Pax-2 by WT1 during normal kidney development. Development 121, 867–875 (1995).
Donovan, M, et al. Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev. Genet. 24, 252–262 (1999).
Brophy, P. D., Ostrom, L., Lang, K. M. & Dressler, G. R. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128, 4747–4756 (2001).Shows that Pax2 might participate in the initiation of kidney development by controlling ureteric-bud formation by Gdnf.
Moore, M. W. et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 (1996).
Sanchez, M. P. et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382, 70–73 (1996).
Pichel, J. G. et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382, 73–76 (1996).
Pepicelli, C. V., Kispert, A., Rowitch, D. H. & McMahon, A. P. GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev. Biol. 192, 193–198 (1997).
Sainio, K. et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124, 4077–4087 (1997).
Xu, P. X. et al. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nature Genet. 23, 113–117 (1999).
Abdelhak, S. et al. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nature Genet. 15, 157–614 (1997).
Nishinakamura, R. et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128, 3105–3115 (2001).
Kume, T., Deng, K. & Hogan, B. L. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127, 1387–1395 (2000).Reports that the Foxc genes are important in controlling where kidney development occurs, possibly by controlling Gdnf expression.
Airaksinen, M. S. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. Nature Rev. Neurosci. 3, 383–394 (2002).
Pachnis, V., Mankoo, B. & Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, 1005–1017 (1993).
Schuchardt, A., D'Agati, V., Larsson-Blomberg, L., Costantini, F. & Pachnis, V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 (1994).In this study, the inactivation of Ret revealed its essential role in early kidney development.
Durbec, P. et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature 381, 789–793 (1996).
Vega, Q. C., Worby, C. A., Lechner, M. S., Dixon, J. E. & Dressler, G. R. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc. Natl Acad. Sci. USA 93, 10657–10661 (1996).
Enomoto, H. et al. GFR α1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21, 317–324 (1998).
de Graaff, E. et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 15, 2433–2444 (2001).
Tang, M. J., Worley, D., Sanicola, M. & Dressler, G. R. The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J. Cell Biol. 142, 1337–1345 (1998).
Tang, M. J., Cai, Y., Tsai, S. J., Wang, Y. K. & Dressler, G. R. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev. Biol. 243, 128–136 (2002).
Sakurai, H., Bush, K. T. & Nigam, S. K. Identification of pleiotrophin as a mesenchymal factor involved in ureteric bud branching morphogenesis. Development 128, 3283–3293 (2001).
Miyazaki, Y., Oshima, K., Fogo, A., Hogan, B. L. & Ichikawa, I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 105, 863–873 (2000).
Raatikainen-Ahokas, A., Hytonen, M., Tenhunen, A., Sainio, K. & Sariola, H. BMP-4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev. Dyn. 217, 146–158 (2000).
Perrimon, N. & Bernfield, M. Cellular functions of proteoglycans – an overview. Semin. Cell Dev. Biol. 12, 65–67 (2001).
Selleck, S. B. Proteoglycans and pattern formation: sugar biochemistry meets developmental genetics. Trends Genet. 16, 206–212 (2000).
Davies, J., Lyon, M., Gallagher, J. & Garrod, D. Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development 121, 1507–1517 (1995).
Bullock, S. L., Fletcher, J. M., Beddington, R. S. & Wilson, V. A. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 12, 1894–1906 (1998).
Kobayashi, M., Habuchi, H., Yoneda, M., Habuchi, O. & Kimata, K. Molecular cloning and expression of Chinese hamster ovary cell heparan-sulfate 2-sulfotransferase. J. Biol. Chem. 272, 13980–13985 (1997).
Kispert, A., Vainio, S., Shen, L., Rowitch, D. H. & McMahon, A. P. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development 122, 3627–3637 (1996).
Merry, C. L. et al. The molecular phenotype of heparan sulfate in the Hs2st−/− mutant mouse. J. Biol. Chem. 276, 35429–35434 (2001).
Cano-Gauci, D. F. et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J. Cell Biol. 146, 255–264 (1999).
Grisaru, S. & Rosenblum, N. D. Glypicans and the biology of renal malformations. Pediatr. Nephrol. 16, 302–306 (2001).
Grisaru, S., Cano-Gauci, D., Tee, J., Filmus, J. & Rosenblum, N. D. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev. Biol. 231, 31–46 (2001).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Karumanchi, S. A. et al. Cell surface glypicans are low-affinity endostatin receptors. Mol. Cell 7, 811–822 (2001).
O'Reilly, M. S. et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285 (1997).
Lin, Y. et al. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development 128, 1573–1585 (2001).
Karihaloo, A. et al. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc. Natl Acad. Sci. USA 98, 12509–12514 (2001).
Clark, A. T. & Bertram, J. F. Molecular regulation of nephron endowment. Am. J. Physiol. 276, F485–F497 (1999).
Miyamoto, N., Yoshida, M., Kuratani, S., Matsuo, I. & Aizawa, S. Defects of urogenital development in mice lacking Emx2. Development 124, 1653–1664 (1997).
Huelsken, J. & Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11, 547–553 (2001).
Yamaguchi, T. P. Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 11, R713–R724 (2001).
Herzlinger, D., Qiao, J., Cohen, D., Ramakrishna, N. & Brown, A. M. Induction of kidney epithelial morphogenesis by cells expressing Wnt-1. Dev. Biol. 166, 815–818 (1994).The first demonstration that Wnt signalling might be important in kidney-tubule induction and is sufficient to induce kidney tubules in vitro.
Stark, K., Vainio, S., Vassileva, G. & McMahon, A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).The first paper to demonstrate the essential role of Wnt signals in nephrogenesis.
Vainio, S. J. & Uusitalo, M. S. A road to kidney tubules via the Wnt pathway. Pediatr. Nephrol. 15, 151–156 (2000).
De Strooper, B. & Annaert, W. Where Notch and Wnt signaling meet. The presenilin hub. J. Cell Biol. 152, F17–F20 (2001).
McCright, B. et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128, 491–502 (2001).
Kispert, A., Vainio, S. & McMahon, A. P. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125, 4225–4234 (1998).This study demonstrated that Wnt4 signalling is important in inducing nephrogenesis in vitro.
Itäranta, P. et al. Wnt-6 is expressed in the ureter bud and induces kidney tubule development in vitro. Genesis 32, 259–268 (2002).
Stark, M. R. et al. Frizzled-4 expression during chick kidney development. Mech. Dev. 98, 121–125 (2000).
Leyns, L., Bouwmeester, T., Kim, S. H., Piccolo, S. & De Robertis, E. M. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88, 747–756 (1997).
Lescher, B., Haenig, B. & Kispert, A. sFRP-2 is a target of the Wnt-4 signaling pathway in the developing metanephric kidney. Dev. Dyn. 213, 440–451 (1998).
Yoshino, K. et al. Secreted Frizzled-related proteins can regulate metanephric development. Mech. Dev. 102, 45–55 (2001).
Montesano, R., Matsumoto, K., Nakamura, T. & Orci, L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67, 901–908 (1991).
Sudarsan, V., Pasalodos-Sanchez, S., Wan, S., Gampel, A. & Skaer, H. A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila. Development 129, 935–944 (2002).
Ainsworth, C., Wan, S. & Skaer, H. Coordinating cell fate and morphogenesis in Drosophila renal tubules. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 931–937 (2000).
Hogan, B. L. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 6, 432–438 (1996).
Dudley, A. T. & Robertson, E. J. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 208, 349–362 (1997).
Dudley, A. T., Lyons, K. M. & Robertson, E. J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9, 2795–2807 (1995).
Luo, G. et al. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9, 2808–2820 (1995).
Reddi, A. H. Bone morphogenetic proteins and skeletal development: the kidney–bone connection. Pediatr. Nephrol. 14, 598–601 (2000).
Al-Awqati, Q. & Oliver, J. A. Stem cells in the kidney. Kidney Int. 61, 387–395 (2002).
Dudley, A. T., Godin, R. E. & Robertson, E. J. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev. 13, 1601–1613 (1999).Reports that the nephrogenic zone and stromal zone might exchange signals and regulate kidney development.
Hatini, V., Huh, S. O., Herzlinger, D., Soares, V. C. & Lai, E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 10, 1467–1478 (1996).This knockout of Bf2 indicates that the stromal cells are essential for kidney development.
Mendelsohn, C., Batourina, E., Fung, S., Gilbert, T. & Dodd, J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126, 1139–1148 (1999).
Batourina, E. et al. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nature Genet. 27, 74–78 (2001).Reports that Ret can rescue the kidney defect in Rar α/ Rar β double-knockout embryos, indicating that stromal cells might signal to regulate ureteric-bud activity.
Qiao, J. et al. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126, 547–554 (1999).
Kawakami, Y. et al. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104, 891–900 (2001).
Lin, Y. et al. Induction of ureter branching as a response to Wnt-2b signaling during early kidney organogenesis. Dev. Dyn. 222, 26–39 (2001).
Serluca, F. C. & Fishman, M. C. Pre-pattern in the pronephric kidney field of zebrafish. Development 128, 2233–2241 (2001).
Drummond, I. A. et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125, 4655–4667 (1998).
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001).
Matsumoto, K., Yoshitomi, H., Rossant, J. & Zaret, K. S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563 (2001).
Stuart, R. O., Bush, K. T. & Nigam, S. K. Changes in global gene expression patterns during development and maturation of the rat kidney. Proc. Natl Acad. Sci. USA 98, 5649–5654 (2001).
Valerius, M. T., Patterson, L. T., Witte, D. P. & Potter, S. S. Microarray analysis of novel cell lines representing two stages of metanephric mesenchyme differentiation. Mech. Dev. 112, 219–232 (2002).
Acknowledgements
We thank S. Kuure (Department of Biomedicine, Biomedicum, University of Helsinki, Finland) and P. Itäranta (Biocenter Oulu and Department of Biochemistry, Linnanmaa, University of Oulu, Oulu, Finland) for the images in figure 3. We acknowledge support from the Academy of Finland, Sigrid Jusélius Foundation and EU programme. We apologize for the failure to cite many important contributions to the field owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Flybase
LocusLink
OMIM
Simpson–Golabi–Behmel syndrome
FURTHER INFORMATION
Encyclopedia of Life Sciences
kidney and renal tract: developmental disorders
The Kidney Development Database
National Institute of Diabetes & Digestive & Kidney Diseases
Glossary
- WOLFFIAN DUCT
-
A duct that originates from the mesonephros, which gives rise to the ureteric bud of the metanephric kidney. The duct persists in males to contribute to parts of the gonad, but regresses in females.
- CHORIOALLANTOIC MEMBRANE
-
A membrane that surrounds bird embryos, which lies adjacent to the egg shell and contributes to gas exchange.
- INTERMEDIATE MESODERM
-
A specific region of embryonic mesoderm that forms the kidney and the sex organs.
- METANEPHRIC BLASTEMA
-
Loosely organized mesenchymal cells that form nephrons in the kidney.
- NEPHROGENIC MESENCHYME
-
Mesenchymal cells adjacent to the tips of the branching ureteric bud that will form the nephrons.
- STROMA
-
Connective tissue that is made up of cells, such as fibroblasts, and matrix, such as collagen.
- NEPHRON
-
The functional unit of the adult kidney that contains the organ's secretory and excretory parts.
- GLOMERULUS
-
The network of blood capillaries in the cup-like end (Bowman's capsule) of the nephron, where waste products are filtered from the blood into the kidney tubule.
- COLLECTING DUCT
-
Structures of the collecting-duct system that drain the nephrons of urine and that derive from the branched ureteric bud.
- RENAL PELVIS
-
A funnel-shaped structure that is formed at one end by the expanded upper portion of the ureter and at the other by the union of the calyxes of the kidney, which receive urine from the collecting ducts. Urine collected here is funnelled through to the ureter, to be collected in the bladder and then excreted.
- ENDOTHELIAL CELLS
-
Flattened cells that grow in a single layer and line blood vessels.
- HOMEOBOX
-
A 180-base-pair sequence that is present in many developmental genes of animals and plants. It encodes a DNA-binding helix–turn–helix motif, indicating that homeobox-containing gene products function as transcription factors.
- GLYCOSAMINOGLYCANS
-
(GAGs). Polysaccharides that comprise repeating disaccharide units of amino-sugar derivatives that are covalently bonded to proteins to function as proteoglycans. Four types of GAG exist according to their sugar residues, and sulphate group numbers and location: chondroitan sulphate and dermatan sulphate; heparin sulphate and heparin; keratan sulphate and hyaluronan.
- HEPARAN SULPHATE
-
A sulphated polysaccharide that is found in cell-surface proteoglycans and is structurally similar to heparin. Heparan sulphates are highly heterogeneous, with different lengths of saccharide chain, levels of sulphation and core carbohydrate sequences.
Rights and permissions
About this article
Cite this article
Vainio, S., Lin, Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet 3, 533–543 (2002). https://doi.org/10.1038/nrg842
Issue Date:
DOI: https://doi.org/10.1038/nrg842
This article is cited by
-
Deciphering the mutation spectrum in south Indian children with congenital anomalies of the kidney and urinary tract
BMC Nephrology (2022)
-
Constitutive depletion of Slc34a2/NaPi-IIb in rats causes perinatal mortality
Scientific Reports (2021)
-
Post-translational modifications by SIRT3 de-2-hydroxyisobutyrylase activity regulate glycolysis and enable nephrogenesis
Scientific Reports (2021)
-
FAM20B-catalyzed glycosaminoglycans control murine tooth number by restricting FGFR2b signaling
BMC Biology (2020)
-
The genetic changes of Wilms tumour
Nature Reviews Nephrology (2019)