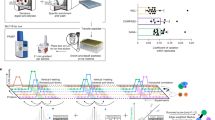
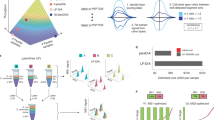
Abstract
The elucidation of protein–protein interaction networks is a crucial task in the postgenomic era. In this protocol, we describe our approach to discover protein–protein interactions using the surface plasmon resonance technique coupled to mass spectrometry (MS). A peptide or a protein is immobilized on a sensor chip and then exposed to brain extracts injected through the surface of the chip by a microfluidic system. The interactions between the immobilized ligand and the extracts can be monitored in real time. Proteins interacting with the peptide/protein are recovered, trypsinated and identified using MS. The data obtained are searched against a sequence database using the Mascot 2.1 software. Control experiments using blank sensor chips and/or randomized peptides are carried out to exclude nonspecific interactors. The protocol can be carried out in <3 days. Other methods, such as yeast two-hybrid systems or pull-down approaches followed by MS, are widely used to screen protein–protein interactions. However, as the yeast two-hybrid system requires protein interactions in the nucleus of yeast, proteins that are abundant in other compartments may not be detected. Pull-down approaches based on immunoprecipitation can be used to study endogenous proteins but they require specific antibodies. The protocol presented here does not require the specific labeling or modification of proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Moszczynska, A. et al. Parkin disrupts the alpha-synuclein/dopamine transporter interaction: consequences toward dopamine-induced toxicity. J. Mol. Neurosci. 32, 217–227 (2007).
Kim, M. et al. Impairment of microtubule system increases alpha-synuclein aggregation and toxicity. Biochem. Biophys. Res. Commun. 365, 628–635 (2008).
Aisenbrey, C. et al. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur. Biophys. J. 37, 247–255 (2008).
Nilsson, A. et al. Increased striatal mRNA and protein levels of the immunophilin FKBP-12 in experimental Parkinson's disease and identification of FKBP-12-binding proteins. J. Proteome Res. 6, 3952–3961 (2007).
Ohman, E. et al. Use of surface plasmon resonance coupled with mass spectrometry reveals an interaction between the voltage-gated sodium channel type X alpha-subunit and Caveolin-1. J. Proteome Res. 7, 5333–5338 (2008).
Poon, W.Y., Malik-Hall, M., Wood, J.N. & Okuse, K. Identification of binding domains in the sodium channel Na(V)1.8 intracellular N-terminal region and annexin II light chain p11. FEBS. Lett. 558, 114–118 (2004).
Svenningsson, P. et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311, 77–80 (2006).
McDonnell, J.M. Surface plasmon resonance: towards an understanding of the mechanisms of biological molecular recognition. Curr. Opin. Chem. Biol. 5, 572–577 (2001).
Mattei, B., Borch, J. & Roepstorff, P. Surface plasmon resonance coupled with MS is a practical tool for identifying and characterizing protein interactions. Anal. Chem. 19A–25A (2004).
Nguyen, B., Tanious, F.A. & Wilson, W.D. Biosensor-surface plasmon resonance: quantitative analysis of small molecule–nucleic acid interactions. Methods 42, 150–161 (2007).
Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 108, 462–493 (2008).
Hoa, X.D., Kirk, A.G. & Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: a review of recent progress. Biosens. Bioelectron. 23, 151–160 (2007).
Visser, N.F., Scholten, A., van den Heuvel, R.H. & Heck, A.J. Surface-plasmon-resonance-based chemical proteomics: efficient specific extraction and semiquantitative identification of cyclic nucleotide-binding proteins from cellular lysates by using a combination of surface plasmon resonance, sequential elution and liquid chromatography–tandem mass spectrometry. Chembiochem 8, 298–305 (2007).
Baerga-Ortiz, A., Rezaie, A.R. & Komives, E.A. Electrostatic dependence of the thrombin–thrombomodulin interaction. J. Mol. Biol. 296, 651–658 (2000).
Laich, A. & Sim, R.B. Complement C4bC2 complex formation: an investigation by surface plasmon resonance. Biochim. Biophys. Acta. 1544, 96–112 (2001).
De Crescenzo, G., Grothe, S., Lortie, R., Debanne, M.T. & O'Connor-McCourt, M. Real-time kinetic studies on the interaction of transforming growth factor alpha with the epidermal growth factor receptor extracellular domain reveal a conformational change model. Biochemistry 39, 9466–9476 (2000).
Fang, Y., Li, G. & Ferrie, A.M. Non-invasive optical biosensor for assaying endogenous G protein-coupled receptors in adherent cells. J. Pharmacol. Toxicol. Methods 55, 314–322 (2007).
Zhou, M. & Veenstra, T. Mass spectrometry: m/z 1983–2008. Biotechniques 44, 667–668, 670 (2008).
Ashcroft. Ionization Methods in Organic Mass Spectrometry (Royal Society of Chemistry, London, UK, 1997).
Ho, C. et al. Electrospray ionisation mass spectrometry: principles and clinical applications. Clin. Biochem. Rev. 24, 3–12 (2003).
Vogeser, M. & Parhofer, K.G. Liquid chromatography tandem-mass spectrometry (LC-MS/MS)—technique and applications in endocrinology. Exp. Clin. Endocrinol. Diabetes 115, 559–570 (2007).
Perkins, D.N., Pappin, D.J., Creasy, D.M. & Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
Krone, J.R., Nelson, R.W., Dogruel, D., Williams, P. & Granzow, R. BIA/MS: interfacing biomolecular interaction analysis with mass spectrometry. Anal. Biochem. 244, 124–132 (1997).
Nelson, R.W., Krone, J.R. & Jansson, O. Surface plasmon resonance biomolecular interaction analysis mass spectrometry. 1. Chip-based analysis. Anal. Chem. 69, 4363–4368 (1997).
Nedelkov, D. & Nelson, R.W. Analysis of human urine protein biomarkers via biomolecular interaction analysis mass spectrometry. Am. J. Kidney Dis. 38, 481–487 (2001).
Chatelier, R.C., Gengenbach, T.R., Griesser, H.J., Brigham-Burke, M. & O'Shannessy, D.J. A general method to recondition and reuse BIAcore sensor chips fouled with covalently immobilized protein/peptide. Anal. Biochem. 229, 112–118 (1995).
Buijs, J. & Franklin, G.C. SPR-MS in functional proteomics. Brief. Funct. Genomic. Proteomic. 4, 39–47 (2005).
Borch, J. & Roepstorff, P. Screening for enzyme inhibitors by surface plasmon resonance combined with mass spectrometry. Anal. Chem. 76, 5243–5248 (2004).
Larsericsdotter, H. et al. Optimizing the surface plasmon resonance/mass spectrometry interface for functional proteomics applications: how to avoid and utilize nonspecific adsorption. Proteomics 6, 2355–2364 (2006).
Murphy, J.C., Winters, M.A. & Sagar, S.L. Large-scale, nonchromatographic purification of plasmid DNA. Methods Mol. Med. 127, 351–362 (2006).
Quest, A.F., Leyton, L. & Parraga, M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem. Cell Biol. 82, 129–144 (2004).
Kawabe, J., Okumura, S., Nathanson, M.A., Hasebe, N. & Ishikawa, Y. Caveolin regulates microtubule polymerization in the vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 342, 164–169 (2006).
Acknowledgements
This work was supported by the Swedish Research Council (Vetenskapsrådet, VR), the French Foundation for Medical Research (Fondation pour la Recherche Médicale FRM) and the Torgny and Ragnar Söderberg's Foundation (Söderberg Stiftelse).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure 1 Program to immobilize a peptide on a carboxymethylated sensor chip.
This program consists of a sequence of commands controlling the immobilization experiment. A translation of these commands can be found in the procedure. Exclamation points allow adding some comments which are not included in the operation. (PPT 128 kb)
Supplementary Figure 2 Program to bind and recover proteins against a peptide on a carboxymethylated sensor chip.
This program consists of a sequence of commands controlling the recovery experiments in the instrument. A translation of these commands can be found in the procedure. Exclamation points allow adding some comments which are not included in the operation. (PPT 130 kb)
Supplementary Figure 3 Program to bind and recover proteins against a peptide on a carboxymethylated chip in the surface prep unit.
This program consists of a sequence of commands controlling the recovery experiments in the surface prep unit. A translation of these commands can be found in the procedure. Exclamation points allow adding some comments which are not included in the operation. (PPT 130 kb)
Supplementary Figure 4 Mass spectra assigned to the peptide ASFTTFTVTK derived from Caveolin-1 (P41350).
The spectra contain the parent mass, charge and observed fragmentation pattern in the form of peak list. Monoisotopic mass of neutral peptide Mr (calc): 1101.57. Ions Score: 64; Expect: 1.7e-005. Matches (Bold Red): 12/86 fragment ions using 24 most intense peaks (modified and reproduced with permission from 5). All animal experiments are performed according to the local ethical committee at the Karolinska Institute (Application N282/06) (PPT 91 kb)
Rights and permissions
About this article
Cite this article
Madeira, A., Öhman, E., Nilsson, A. et al. Coupling surface plasmon resonance to mass spectrometry to discover novel protein–protein interactions. Nat Protoc 4, 1023–1037 (2009). https://doi.org/10.1038/nprot.2009.84
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.84
This article is cited by
-
Hybrid methods of surface plasmon resonance coupled to mass spectrometry for biomolecular interaction analysis
Analytical and Bioanalytical Chemistry (2019)
-
Nanoscale monitoring of drug actions on cell membrane using atomic force microscopy
Acta Pharmacologica Sinica (2015)
-
Molecular screening of cancer-derived exosomes by surface plasmon resonance spectroscopy
Analytical and Bioanalytical Chemistry (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.