Abstract
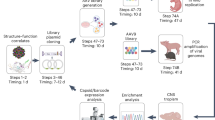
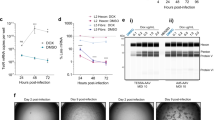
High-capacity adenoviral vectors (HC-AdVs) lacking all viral coding sequences were shown to result in long-term transgene expression and phenotypic correction in small and large animal models. It has been established that HC-AdVs show significantly reduced toxicity profiles compared with early-generation adenoviral vectors. Furthermore, with capsid-modified HC-AdV becoming available, we are just starting to understand the full potential of this vector system. However, for many researchers, the wide-scale use of HC-AdV is hampered by labor-intensive and complex production procedures. Herein, we provide a feasible and detailed protocol for efficient generation of HC-AdV. We introduce an efficient cloning strategy for the generation of recombinant HC-AdV vector genomes. Infection and amplification of the HC-AdV are performed in a spinner culture system. For purification, we routinely apply cesium chloride gradients. Finally, we describe various methods for establishing vector titers. Generation of high-titer HC-AdV can be achieved in 3 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Luo, J. et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 (2007).
Yang, Y., Ertl, H.C. & Wilson, J.M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1, 433–442 (1994).
Hardy, S., Kitamura, M., Harris-Stansil, T., Dai, Y. & Phipps, M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 71, 1842–1849 (1997).
Hartigan-O'Connor, D., Amalfitano, A. & Chamberlain, J.S. Improved production of gutted adenovirus in cells expressing adenovirus preterminal protein and DNA polymerase. J. Virol. 73, 7835–7841 (1999).
Fisher, K.J., Choi, H., Burda, J., Chen, S.J. & Wilson, J.M. Recombinant adenovirus deleted of all viral genes for gene therapy of cystic fibrosis. Virology 217, 11–22 (1996).
Parks, R.J. et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93, 13565–13570 (1996).
Zhang, Y. & Bergelson, J.M. Adenovirus receptors. J. Virol. 79, 12125–12131 (2005).
Benihoud, K., Yeh, P. & Perricaudet, M. Adenovirus vectors for gene delivery. Curr. Opin. Biotechnol. 10, 440–447 (1999).
Kozarsky, K.F. & Wilson, J.M. Gene therapy: adenovirus vectors. Curr. Opin. Genet. Dev. 3, 499–503 (1993).
Wickham, T.J., Roelvink, P.W., Brough, D.E. & Kovesdi, I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat. Biotechnol. 14, 1570–1573 (1996).
Kawano, R. et al. Transduction of full-length dystrophin to multiple skeletal muscles improves motor performance and life span in utrophin/dystrophin double knockout mice. Mol. Ther. 16, 825–831 (2008).
Deol, J.R. et al. Successful compensation for dystrophin deficiency by a helper-dependent adenovirus expressing full-length utrophin. Mol. Ther. 15, 1767–1774 (2007).
Gilbert, R. et al. Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum. Mol. Genet. 12, 1287–1299 (2003).
Brown, B.D. et al. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood 103, 804–810 (2004).
McCormack, W.M. Jr. et al. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 4, 1218–1225 (2006).
Cerullo, V. et al. Correction of murine hemophilia A and immunological differences of factor VIII variants delivered by helper-dependent adenoviral vectors. Mol. Ther. 15, 2080–2087 (2007).
Koeberl, D.D. et al. Efficacy of helper-dependent adenovirus vector-mediated gene therapy in murine glycogen storage disease type Ia. Mol. Ther. 15, 1253–1258 (2007).
Oka, K. et al. Sustained phenotypic correction in a mouse model of hypoalphalipoproteinemia with a helper-dependent adenovirus vector. Gene. Ther. 14, 191–202 (2007).
Toietta, G. et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA 102, 3930–3935 (2005).
Mian, A. et al. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol. Ther. 10, 492–499 (2004).
Nomura, S. et al. Low-density lipoprotein receptor gene therapy using helper-dependent adenovirus produces long-term protection against atherosclerosis in a mouse model of familial hypercholesterolemia. Gene. Ther. 11, 1540–1548 (2004).
Brunetti-Pierri, N. et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene. Ther. 16, 811–820 (2005).
Ehrhardt, A. et al. A gene-deleted adenoviral vector results in phenotypic correction of canine hemophilia B without liver toxicity or thrombocytopenia. Blood 102, 2403–2411 (2003).
Ehrhardt, A. & Kay, M.A. A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo . Blood 99, 3923–3930 (2002).
Lamartina, S. et al. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J. Gene. Med. 9, 862–874 (2007).
Butti, E. et al. IL4 gene delivery to the CNS recruits regulatory T cells and induces clinical recovery in mouse models of multiple sclerosis. Gene. Ther. 15, 504–515 (2008).
Brunetti-Pierri, N. et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol. Ther. 15, 732–740 (2007).
Jager, L. & Ehrhardt, A. Emerging adenoviral vectors for stable correction of genetic disorders. Curr. Gene. Ther. 7, 272–283 (2007).
Soifer, H. et al. A novel, helper-dependent, adenovirus-retrovirus hybrid vector: stable transduction by a two-stage mechanism. Mol. Ther. 5, 599–608 (2002).
Picard-Maureau, M. et al. Foamy virus-adenovirus hybrid vectors. Gene. Ther. 11, 722–728 (2004).
Yant, S.R. et al. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo . Nat. Biotechnol. 20, 999–1005 (2002).
Ehrhardt, A. et al. Somatic integration from an adenoviral hybrid vector into a hot spot in mouse liver results in persistent transgene expression levels in vivo . Mol. Ther. 15, 146–156 (2007).
Lieber, A., Steinwaerder, D.S., Carlson, C.A. & Kay, M.A. Integrating adenovirus-adeno-associated virus hybrid vectors devoid of all viral genes. J. Virol. 73, 9314–9324 (1999).
Recchia, A., Perani, L., Sartori, D., Olgiati, C. & Mavilio, F. Site-specific integration of functional transgenes into the human genome by adeno/AAV hybrid vectors. Mol. Ther. 10, 660–670 (2004).
Kreppel, F. & Kochanek, S. Long-term transgene expression in proliferating cells mediated by episomally maintained high-capacity adenovirus vectors. J. Virol. 78, 9–22 (2004).
Dorigo, O. et al. Development of a novel helper-dependent adenovirus–Epstein–Barr virus hybrid system for the stable transformation of mammalian cells. J Virol 78, 6556–6566 (2004).
Suzuki, K. et al. Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors. Proc. Natl. Acad. Sci. USA 105, 13781–13786 (2008).
Ohbayashi, F. et al. Correction of chromosomal mutation and random integration in embryonic stem cells with helper-dependent adenoviral vectors. Proc. Natl. Acad. Sci. USA 102, 13628–13633 (2005).
Cregan, S.P. et al. Helper-dependent adenovirus vectors: their use as a gene delivery system to neurons. Gene. Ther. 7, 1200–1209 (2000).
Wang, H., Cao, H., Wohlfahrt, M., Kiem, H.P. & Lieber, A. Tightly regulated gene expression in human hematopoietic stem cells after transduction with helper-dependent Ad5/35 vectors. Exp. Hematol. 36, 823–831 (2008).
Balamotis, M.A., Huang, K. & Mitani, K. Efficient delivery and stable gene expression in a hematopoietic cell line using a chimeric serotype 35 fiber pseudotyped helper-dependent adenoviral vector. Virology 324, 229–237 (2004).
Cerullo, V. et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15, 378–385 (2007).
Zhu, J., Huang, X. & Yang, Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J. Virol. 81, 3170–3180 (2007).
Koehler, D.R. et al. Aerosol delivery of an enhanced helper-dependent adenovirus formulation to rabbit lung using an intratracheal catheter. J. Gene. Med. 7, 1409–1420 (2005).
Croyle, M.A., Yu, Q.C. & Wilson, J.M. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene. Ther. 11, 1713–1722 (2000).
Ruzsics, Z. et al. Transposon-assisted cloning and traceless mutagenesis of adenoviruses: Development of a novel vector based on species D. J. Virol. 80, 8100–8113 (2006).
Umana, P. et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 19, 582–585 (2001).
Ng, P., Beauchamp, C., Evelegh, C., Parks, R. & Graham, F.L. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 3, 809–815 (2001).
Meneses-Acosta, A. et al. Development of a suspension serum-free helper-dependent adenovirus production system and assessment of co-infection conditions. J. Virol. Methods 148, 106–114 (2008).
Zhou, H. et al. A Cre-expressing cell line and an E1/E2a double-deleted virus for preparation of helper-dependent adenovirus vector. Mol. Ther. 3, 613–622 (2001).
Barjot, C., Hartigan-O'Connor, D., Salvatori, G., Scott, J.M. & Chamberlain, J.S. Gutted adenoviral vector growth using E1/E2b/E3-deleted helper viruses. J. Gene. Med. 4, 480–489 (2002).
Ng, P., Parks, R.J. & Graham, F.L. Preparation of helper-dependent adenoviral vectors. Methods Mol. Med. 69, 371–388 (2002).
Sandig, V. et al. Optimization of the helper-dependent adenovirus system for production and potency in vivo . Proc. Natl. Acad. Sci. USA 97, 1002–1007 (2000).
Rauschhuber, C., Xu, H., Salazar, F.H., Marion, P.L. & Ehrhardt, A. Exploring gene-deleted adenoviral vectors for delivery of short hairpin RNAs and reduction of hepatitis B virus infection in mice. J. Gene. Med. 10, 878–889 (2008).
Palmer, D. & Ng, P. Improved system for helper-dependent adenoviral vector production. Mol. Ther. 8, 846–852 (2003).
Hillgenberg, M., Schnieders, F., Loser, P. & Strauss, M. System for efficient helper-dependent minimal adenovirus construction and rescue. Hum. Gene. Ther. 12, 643–657 (2001).
Shi, C.X., Graham, F.L. & Hitt, M.M. A convenient plasmid system for construction of helper-dependent adenoviral vectors and its application for analysis of the breast-cancer-specific mammaglobin promoter. J. Gene. Med. 8, 442–451 (2006).
Toietta, G. et al. Generation of helper-dependent adenoviral vectors by homologous recombination. Mol. Ther. 5, 204–210 (2002).
Mizuguchi, H. & Kay, M.A. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene. Ther. 9, 2577–2583 (1998).
Parks, R.J. & Graham, F.L. A helper-dependent system for adenovirus vector production helps define a lower limit for efficient DNA packaging. J. Virol. 71, 3293–3298 (1997).
Bett, A.J., Prevec, L. & Graham, F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 67, 5911–5921 (1993).
Sambrook, J. & Russell, D.W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2001).
Offringa, R., Kwappenberg, K., Rabelink, M., Rea, D. & Hoeben, R. Adenoviral transduction of dendritic cells. Methods Mol. Med. 109, 83–96 (2005).
Ugai, H. et al. Stability of a recombinant adenoviral vector: optimization of conditions for storage, transport and delivery. Jpn. J. Cancer. Res. 93, 598–603 (2002).
Nyberg-Hoffman, C. & Aguilar-Cordova, E. Instability of adenoviral vectors during transport and its implication for clinical studies. Nat. Med. 5, 955–957 (1999).
Puntel, M. et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum. Gene. Ther. 17, 531–544 (2006).
Palmer, D.J. & Ng, P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol. Ther. 10, 792–798 (2004).
Haase, S.B. & Calos, M.P. Replication control of autonomously replicating human sequences. Nucleic Acids. Res. 19, 5053–5058 (1991).
Acknowledgements
This work was supported by DFG grants SFB455 and SPP1230 and the Wilhelm Sander Stiftung to A.E. We thank Philip Ng for helpful discussions and advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jager, L., Hausl, M., Rauschhuber, C. et al. A rapid protocol for construction and production of high-capacity adenoviral vectors. Nat Protoc 4, 547–564 (2009). https://doi.org/10.1038/nprot.2009.4
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.4
This article is cited by
-
Preferential expression of SCN1A in GABAergic neurons improves survival and epileptic phenotype in a mouse model of Dravet syndrome
Journal of Molecular Medicine (2023)
-
Human adenovirus type 17 from species D transduces endothelial cells and human CD46 is involved in cell entry
Scientific Reports (2018)
-
CRISPR/Cas9 delivery with one single adenoviral vector devoid of all viral genes
Scientific Reports (2017)
-
Use of luciferase probes to measure ATP in living cells and animals
Nature Protocols (2017)
-
Hepatocytic expression of human sodium-taurocholate cotransporting polypeptide enables hepatitis B virus infection of macaques
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.