Abstract
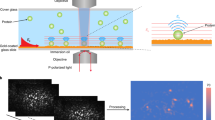
Immuno-spin trapping is a highly sensitive method for detecting DNA radicals in biological systems. This technique involves three main steps: (i) in situ and real-time trapping of DNA radicals with the nitrone spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), thus forming DMPO–DNA nitrone adducts (referred to here as nitrone adducts); (ii) purification of nitrone adducts; and (iii) analysis of nitrone adducts by heterogeneous immunoassays using Abs against DMPO. In experiments, DMPO is added prior to the formation of free radicals. It diffuses easily through all cell compartments and is present when DNA free radicals are formed as a result of oxidative damage. Due to its low toxicity, DMPO can be used in cells at high enough concentrations to out-compete the normal reactions of DNA radicals, thus ensuring a high yield of DNA nitrone adducts. Because both protein and DNA nitrone adducts are formed, it is important that the DNA be pure in order to avoid misinterpretations. Depending on the model under study, this protocol can be completed in as few as 6 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Halliwell, B. & Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 142, 231–255 (2004).
Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Halliwell, B. & Gutteridge, J.M. The chemistry of oxygen radicals and other oxygen-derived species. In Free Radicals in Biology and Medicine 20–66 (Oxford Univ. Press Inc., New York, USA, 1985).
Gracy, R.W., Talent, J.M., Kong, Y. & Conrad, C.C. Reactive oxygen species: the unavoidable environmental insult? Mutat. Res. 428, 17–22 (1999).
Winyard, P.G., Moody, C.J. & Jacob, C. Oxidative activation of antioxidant defence. Trends Biochem. Sci. 30, 453–461 (2005).
Imlay, J.A., Chin, S.M. & Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro . Science 240, 640–642 (1988).
Cooke, M.S., Evans, M.D., Dizdaroglu, M. & Lunec, J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 17, 1195–1214 (2003).
Cadet, J., Douki, T., Gasparutto, D. & Ravanat, J.-L. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat. Res. 531, 5–23 (2003).
von Sonntag, C. DNA model systems. In The Chemical Basis of Radiation Biology 116–294 (Taylor & Francis Ltd., London, UK, 1987).
Hall, D.B., Holmlin, R.E. & Barton, J.K. Oxidative DNA damage through long-range electron transfer. Nature 382, 731–735 (1996).
Purkayastha, S., Milligan, J.R. & Bernhard, W.A. Correlation of free radical yields with strand break yields produced in plasmid DNA by the direct effect of ionizing radiation. J. Phys. Chem. B 109, 16967–16973 (2005).
von Sonntag, C. The chemistry of free-radical-mediated DNA damage. Basic Life Sci. 58, 287–321 (1991).
Collins, A.R., Cadet, J., Möller, L., Poulsen, H.E. & Viña, J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch. Biochem. Biophys. 423, 57–65 (2004).
Fraga, C.G., Shigenaga, M.K., Park, J.-W., Degan, P. & Ames, B.N. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. USA 87, 4533–4537 (1990).
European Standards Committee on Oxidative DNA Damage (ESCODD). Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic. Biol. Med. 34, 1089–1099 (2003).
Hildenbrand, K. & Schulte-Frohlinde, D. ESR spectra of radicals of single-stranded and double-stranded DNA in aqueous solution. Implications for •OH-induced strand breakage. Free Radic. Res. Commun. 11, 195–206 (1990).
Cullis, P.M., Jones, G.D.D., Symons, M.C.R. & Lea, J.S. Electron transfer from protein to DNA in irradiated chromatin. Nature 330, 773–774 (1987).
Khan, N. et al. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic. Biol. Med. 34, 1473–1481 (2003).
Haseloff, R.F. et al. Cytotoxicity of spin trapping compounds. FEBS Lett. 418, 73–75 (1997).
Schaefer, C.F., Janzen, E.G., West, M.S., Poyer, J.L. & Kosanke, S.D. Blood chemistry changes in the rat induced by high doses of nitronyl free radical spin traps. Free Radic. Biol. Med. 21, 427–436 (1996).
Anzai, K. et al. ESR measurement of rapid penetration of DMPO and DEPMPO spin traps through lipid bilayer membranes. Arch. Biochem. Biophys. 415, 251–256 (2003).
Ho, W.F., Gilbert, B.C. & Davies, M.J. EPR spin-trapping studies of radicals generated from FeII-catalysed degradation of nucleobase, nucleoside, RNA and DNA hydroperoxides. J. Chem. Soc. Perkin Trans. 2, 2525–2531 (1997).
Kuwabara, M., Ohshima, H., Sato, F., Ono, A. & Matsuda, A. Spin-trapping detection of precursors of hydroxyl-radical-induced DNA damage: identification of precursor radicals of DNA strand breaks in oligo(dC)10 and oligo(dT)10 . Biochemistry 32, 10599–10606 (1993).
Maurel, V., Ravanat, J.-L. & Gambarelli, S. Detection of reactive free radicals derived from nucleosides by liquid chromatography coupled to tandem mass spectrometry of DMPO spin trapping adducts. Rapid Commun. Mass Spec. 20, 2235–2242 (2006).
Janzen, E.G., West, M.S., Kotake, Y. & DuBose, C.M. Biological spin trapping methodology. III. Octanol-water partition coefficients of spin-trapping compounds. J. Biochem. Biophys. Methods 32, 183–190 (1996).
Mason, R.P. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic. Biol. Med. 36, 1214–1223 (2004).
Ramirez, D.C. & Mason, R.P. Immuno-spin trapping: detection of protein-centered radicals. In Current Protocols in Toxicology (eds. Costa, L.G., Maines, M.D., Reed, D.J., Sassa, S. & Sipes, I.G.) 17.7.1–17.7.16 (John Wiley & Sons, Hoboken, New Jersey, USA, 2005).
Ramirez, D.C., Gomez-Mejiba, S.E. & Mason, R.P. Immuno-spin trapping of DNA radicals. Nat. Methods 3, 123–127 (2006).
Ramirez, D.C., Chen, Y.-R. & Mason, R.P. Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic. Biol. Med. 34, 830–839 (2003).
Detweiler, C.D. et al. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic. Biol. Med. 33, 364–369 (2002).
Strober, W. Trypan blue exclusion test of cell viability. In Current Protocols in Immunology (eds. Coligan, J.E., Kruisbeek, A.M., Margulies, D.H., Shevach, E.M. & Strober, W.) A3.B1–A3.B2 (John Wiley & Sons, Inc., Hoboken, New Jersey, USA, 1997).
Crowther, J.R. Titration of reagents. In Methods in Molecular Biology: The ELISA Guidebook Vol 149 83–113 (Humana Press Inc., Totowa, New Jersey, USA, 2001).
Hix, S., Kadiiska, M.B., Mason, R.P. & Augusto, O. In vivo metabolism of tert-butyl hydroperoxide to methyl radicals. EPR spin-trapping and DNA methylation studies. Chem. Res. Toxicol. 13, 1056–1064 (2000).
Latour, I., Demoulin, J.B. & Buc-Calderon, P. Oxidative DNA damage by t-butyl hydroperoxide causes DNA single strand breaks which is not linked to cell lysis. A mechanistic study in freshly isolated rat hepatocytes. FEBS Lett. 373, 299–302 (1995).
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Intramural Research Program. D.C.R. is the recipient of a National Institutes of Health Pathway for Independence Award (1 K99 ES015415-01). We acknowledge J. Corbett for her excellent technical assistance, and M. Ehrenshaft and M. Waalkes for their useful comments. We also thank M. Mason and A. Motten for helping in the editing of this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The anti-DMPO serum has been licensed to the following companies: Cayman Chemicals (cat. no. 10006170-1), Alexis Biochemicals (cat. no. ALX-210-530-R100), Abcam (cat. no. ab23702), and Oxford Biomedical Research (cat. no. RT15). Ronald P. Mason receives a small royalty for this license.
Rights and permissions
About this article
Cite this article
Ramirez, D., Gomez-Mejiba, S. & Mason, R. Immuno-spin trapping analyses of DNA radicals. Nat Protoc 2, 512–522 (2007). https://doi.org/10.1038/nprot.2007.5
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.5
This article is cited by
-
Immuno-spin trapping detection of antioxidant/pro-oxidant properties of zinc or selenium on DNA and protein radical formation via hydrogen peroxide
Molecular and Cellular Biochemistry (2015)
-
Methylarsonous acid causes oxidative DNA damage in cells independent of the ability to biomethylate inorganic arsenic
Archives of Toxicology (2014)
-
Metallothionein blocks oxidative DNA damage in vitro
Archives of Toxicology (2013)
-
Measurement of oxidatively generated base damage to nucleic acids in cells: facts and artifacts
Bioanalytical Reviews (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.