Abstract
The nucleus accumbens (NAc) in the ventral striatum integrates many neurochemical inputs including dopamine and serotonin projections from midbrain nuclei to modulate drug reward. Although D1 and D2 dopamine receptors are differentially expressed in the direct and indirect pathway medium spiny neurons (dMSNs and iMSNs, respectively), 5-HT6 receptors are expressed in both pathways, more strongly than anywhere else in the brain, and are an intriguing target for neuropsychiatric disorders. In the present study, we used viral vectors utilizing dynorphin or enkephalin promoters to drive expression of 5-HT6 receptors or green fluorescent protein (GFP) selectively in the dMSNs or iMSNs of the NAc shell. Rats were then trained to self-administer cocaine. Increased 5-HT6 receptor expression in dMSNs did not change any parameter of cocaine self-administration measured. However, increasing 5-HT6 receptors in iMSNs reduced the amount of cocaine self-administered under fixed-ratio schedules, especially at low doses, increased the time to the first response and the length of the inter-infusion interval, but did not alter motivation as measured by progressive ratio ‘break point’ analysis. Modeling of cocaine pharmacokinetics in NAc showed that increased 5-HT6 receptors in iMSNs reduced the rat’s preferred tissue cocaine concentration at each dose. Finally, increased 5-HT6 receptors in iMSNs facilitated conditioned place preference for a low dose of cocaine. We conclude that 5-HT6 receptors in iMSNs of NAcSh increase the sensitivity to the reinforcing properties of cocaine, particularly at low doses, suggesting that these receptors may be a therapeutic target for the treatment of cocaine addiction.
Similar content being viewed by others
INTRODUCTION
Substance use disorders are prevalent neuropsychiatric conditions that are costly on personal and societal levels. A recent estimate holds substance addiction accountable for over 500 billion dollars spent annually within the United States alone (Rehm et al, 2009; US Department of Justice National Drug Intelligence Center, 2011; United States Department of Health and Human Services, 2014). Addiction is thought to involve maladaptations of the neural processes that normally mediate reward learning in neural circuitry converging in the dorsal and ventral striatum (Hyman et al, 2006), which lead to compulsive substance taking. In particular, the nucleus accumbens (NAc) of ventral striatum is crucial to drug reward and may be an appropriate target for future addiction therapies (Stuber et al, 2011). The NAc comprises mostly GABAergic medium spiny neurons (MSNs) which project through two distinct pathways, known as the direct (striatonigral) and indirect (striatopallidal) pathways (Gerfen et al, 1990, 1991; Surmeier et al, 2007). Output via the direct pathway is thought to facilitate actions while the indirect pathway is thought to inhibit actions (Chandra et al, 2015; Macpherson et al, 2014; Yager et al, 2015). The direct pathway MSNs (dMSNs) express mainly D1 dopamine receptors and dynorphin (Dyn), whereas the indirect pathway MSNs (iMSNs) express mainly D2 dopamine receptors and enkephalin (Enk) (Gerfen et al, 1990, 1991; Hikida et al, 2010). The specific functions and mechanisms underlying drug seeking within these pathways are not well understood, but the relative activity in these pathways is likely to have a role in the progression of addiction (Bock et al, 2013; Hikida et al, 2010).
Cocaine’s reinforcing properties involve direct interactions with monoamine transporters including the serotonin transporter (Sora et al, 2001; Uchimura and North, 1990). Serotonin neurons strongly innervate ventral striatum (Dölen et al, 2013; McDevitt et al, 2014; Parsons and Justice, 1993a) and cocaine increases extracellular serotonin in NAc (Andrews and Lucki, 2001; Parsons and Justice, 1993b). By increasing extracellular 5-HT, cocaine enhances signaling at multiple 5-HT receptor subtypes, several of which have been implicated in mediating the reinforcing properties of cocaine in NAc (Müller and Homberg, 2015).
Among the 14 identified serotonin receptors, 5-HT6 receptors are notable for their abundance in striatum, where they are expressed in both dMSNs and iMSNs (Hirst et al, 2003; Tassone et al, 2011; Ward et al, 1995). Some reports found no effect of a systemic 5-HT6 agonist or antagonist on cocaine self-administration (cocaine SA) (Fijal et al, 2010; Frantz et al, 2002; Valentini et al, 2012), while other studies suggested that cocaine reinforcement or reinstatement was regulated by 5-HT6 receptor activity (van Gaalen et al, 2010; Valentini et al, 2012). We previously showed that viral-mediated increases in 5-HT6 receptor expression in NAcSh blocked the acquisition of conditioned place preference (CPP) to cocaine without altering psychomotor sensitization (Ferguson et al, 2008). 5-HT6 receptors also regulate reward motivated learning and the expression of habitual actions (Eskenazi and Neumaier, 2011a, 2011b). In both these studies, 5-HT6 receptors were increased in MSNs generally and were not specifically targeted at the direct or indirect pathway. Recently, we observed that increased striatal 5-HT6 receptor activity had different effects on reward motivated learning using sucrose reinforcers when expression was restricted to either the dMSNs or the iMSNs (Eskenazi et al, 2015a), suggesting that the distribution of the receptors is a key determinant of the behavioral impact of 5-HT6 receptor signaling.
In general, striatal 5-HT6 receptors tend to oppose dopamine’s effects on drug reward (Eskenazi et al, 2015b). Because this might involve opposing the differential activation of both pathways by dopamine, we used phenotype-specific viral vectors to increase expression of 5-HT6 receptors or green fluorescent protein (GFP) selectively in the dMSNs or iMSNs in the NAc shell (NAcSh) of rats. Our original hypothesis was that 5-HT6 receptors in dMSNs and iMSNs would increase and decrease cocaine reinforcement, respectively. However, upon examination of several parameters of cocaine reinforcement and reward, we conclude that increasing 5-HT6 receptors only in iMSNs increases the sensitivity to the reinforcing properties of cocaine, particularly at low doses, suggesting that these receptors may be a target for pharmacological manipulation in the treatment of cocaine addiction.
MATERIALS AND METHODS
Animals
For all experiments, male Long-Evans rats (Charles River, Raleigh, NC) weighing 350–450 g were used. Rats were double-housed for 1 week to acclimate them to the temperature- and humidity-controlled vivarium before the experiment, and were kept under a 12-h light-dark cycle. All experiments were carried out during the light period. Following implantation of intravenous catheters, the rats were housed individually. Food and water were freely available at all times except during the cocaine SA sessions. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee and were conducted in accordance to the guidelines of the ‘Principles of Laboratory Animal Care’ (NIH publication no. 86-23, 1996). A total of 76 rats were used for these experiments, of which 4 were excluded due to failure to learn to self-administer cocaine, 5 were excluded because viral-mediated gene expression was outside the target brain region, and 11 were removed from the study due to health issues (such as lost IV access).
Intravenous Catheter Placement and Intracranial Virus-Mediated Gene Transfer
Jugular catheters were implanted as previously described (Nair et al, 2013). Rats were allowed to recover for 10 days before cocaine SA training. During the recovery and training phases, catheters were flushed every 48 h using sterile gentamicin (0.08 mg/ml). We used replication-deficient herpes-simplex viral (HSV) vectors to increase 5-HT6 receptor expression in either dMSNs or iMSNs of the NAcSh. The experiment utilized four different viral cassettes for this manipulation: two that express fully functional hemagglutinin (HA)-tagged 5-HT6 receptors under either the proenkephalin promoter (Enk-5-HT6) or the prodynorphin promoter (Dyn-5-HT6), and two control viruses that express GFP alone under either the proenkephalin promoter (Enk-GFP) or the prodynorphin promoter (Dyn-GFP). We have previously confirmed that these viral vectors produce HA-tagged 5-HT6 receptors in either enkephalin or dynorphin containing neurons and not glia (Eskenazi et al, 2015a; Ferguson et al, 2011, 2013; Michaelides et al, 2013). HSV vectors were injected at 400 nl/min using surgical procedures previously described (Eskenazi et al, 2015a; Ferguson et al, 2013). The volume of viral vector (2 μl) was chosen based on previous studies in our laboratory to induce discrete infection in the target region (Mitchell and Neumaier, 2008; Mitchell et al, 2007). Using a 10° angle of approach, the NAcSh was targeted using the coordinates relative to bregma: +1.7 mm (anterior-posterior), ±2.3 mm (medial-lateral), and −7.6 mm (dorsal-ventral). To confirm the injection site, rats were perfused as previously described (Eskenazi and Neumaier, 2011a), brains were dissected and post-fixed in 2.5% paraformaldehyde for 6 h, after which they were placed 30% sucrose in PBS. Tissue sections were made on a Leica Jung CM 3000 cryostat and mounted on slides at 40 μm thickness. Accuracy of injection coordinates was confirmed by visualization of the injection needle tracts. Rats with injection sites outside the targeted brain region were excluded from the experiments.
Apparatus
The rats were trained and tested in standard Med Associates operant chambers (Med Associates, Georgia, VT). Each chamber was equipped with two levers located 9 cm above the grid floor. Lever presses on the active lever activated the infusion pump, whereas lever presses on the inactive lever had no programmed response. All chambers were kept in sound-attenuating boxes equipped with fans for temperature regulation and to provide white noise. All chambers were connected to a Med Associates interface, and experimental data were collected using the Med-PC software.
Behavioral Procedures
The procedure consisted of four phases: fixed-ratio cocaine SA (11 days), progressive ratio (PR) (4 days), low-dose responding (3 days), and high-dose responding (3 days).
Cocaine SA
Rats were trained to self-administer cocaine for 2 h/day (two 1-h sessions with a 5-min interval between sessions) for 11 days. Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile injectable 0.9% saline and infused in a volume of 0.1 ml at a dose of 0.75 mg/kg/infusion. Each session started with the turning on of a white house-light and introduction of the levers into the operant chamber. During training, cocaine infusions were earned under a fixed-ratio-1 (FR1), 20 s timeout reinforcement schedule and were accompanied by a compound tone-light cue for 5 s. During the 20-s timeout period, lever presses were recorded but did not result in cocaine delivery. A maximum of 20 cocaine infusions/h was set to prevent cocaine overdose. At the end of each session, the house-light was turned off and the levers were retracted.
Progressive Ratio
Following the FR1 sessions, rats were trained to SA cocaine for 4 days on a PR reinforcement schedule, during which the response requirement to earn a cocaine infusion (0.75 mg/kg/infusion) increased after each infusion earned. The response requirement increased incrementally in accordance with the following equation: Response ratio=(5e (injection number × 0.2)−5) (Richardson and Roberts, 1996). The PR sessions were terminated when the rat failed to receive a cocaine infusion within 1 h.
Cocaine Dose-Response Experiments
Following cocaine SA on FR1 and PR schedules, behavioral responding was measured for 3 days each on a low and a high dose of cocaine. The low-dose testing was done during a 2-h FR1 SA session where each response yielded a cocaine infusion (0.375 mg/kg/infusion). Three days of high-dose (1.5 mg/kg/infusion) FR1 sessions followed immediately after the low-dose period.
Cocaine Pharmacokinetic Modeling
Whole-brain levels of cocaine were modeled using a two-compartment mathematical model for rats receiving i.v. cocaine as previously described (Zimmer et al, 2011), based on an original report that measured extracellular cocaine concentration in the NAc measured by microdialysis (Pan et al, 1991). The average estimated cocaine concentration for each session was calculated between 10 and 120 min after the initiation of the session using the equation:

which gives the estimated cocaine brain concentration (c) by accounting for the dose of cocaine (d), the transport of cocaine between the blood and brain (k=0.233/min), the brain volume (v=0.151/kg), and the removal of cocaine from circulation via redistribution (α=0.642/min) and elimination (β=0.097/min). Group means for each unit dose were calculated from the average values for each animal over the three testing sessions.
Locomotor Activity
On the day following the completion of all cocaine SA behavior, locomotor activity was measured in infrared beam break activity boxes (22 × 45 × 23 cm; San Diego Instruments, San Diego, California) for 30 min in a dimly-lit room. Locomotor activity was analyzed in 3-min bins.
Conditioned Place Preference
A separate cohort of rats began place conditioning trials on the eleventh day after viral infusion using a three-chamber conditioned place preference (CPP) apparatus (Medical Associates, St Albans, VT) comprises two large side chambers (24 × 21 × 21 cm) separated by a small central chamber (12 × 21 × 21 cm). The three chambers differed in lighting (dim, medium, or bright), wall color (white, black, or gray), and floor texture (grid, rod, or solid). Before the onset of the study, light intensities were adjusted so that there was no overall preference by a separate test group of animals for any chamber. We did not use a pre-exposure trial to avoid latent inhibition of associations between the drug effect and the chamber cues (Barot et al, 2007; Tzschentke, 1998). Chamber pairing with drug was randomly assigned in a counterbalanced manner so any pre-existing individual preferences would be randomly distributed.
The first trial of each conditioning day began at 0900–1000 h, with each animal receiving an injection of isotonic saline (1 ml/kg, i.p.). After the injection, animals were confined to one of the side chambers of the CPP apparatus for 15 min. Three hours after the morning trial, animals received cocaine hydrochloride (NIDA 5 mg/kg in 1 ml/kg saline, i.p.), after which they were placed into the other side chamber for 15 min. Conditioning trials were repeated in the same manner for the following 3 days. The day following the three conditioning trials (day 14), animals were tested for CPP. The animal was placed into the central chamber, and after a 3-min habituation period the doors were raised and the animal was allowed to explore the entire apparatus for 15 min. Time spent in each compartment was recorded automatically.
Immunohistochemistry
Floating sections (40 μm) were washed in 0.5% Triton-X/PBS for 10 min, then blocked in 10% normal goat serum (NGS)-Triton-X/PBS for 1 h. Sections were then incubated in 5% NGS-Triton-X/PBS containing HA (1 : 400, rabbit, Cell Signaling) with gentle agitation at 4 °C overnight. Next, sections were rinsed four times in PBS and incubated with Alexa 488-conjugated, goat anti-rabbit secondary antibody (1 : 250, Invitrogen, Carlsbad, CA) for 2 h. Sections were washed three times in PBS, mounted on slides and coverslipped with ProLong Gold Antifade mounting medium (Life Technologies). Images were captured with a Nikon fluorescence microscope and associated ZEN software.
Statistical Analyses
Data from the cocaine SA sessions was collected using the Med PC IV software. Analysis was performed using GraphPad Prism (Version 5.01). Significance for all cocaine SA data was tested with two-way analyses of variance (ANOVAs; with or without repeated measures, as warranted) followed by Bonferroni post hoc tests (Table 1). For analysis involving only two samples we used a two-tailed t-test. For all comparisons, we used an alpha value of 0.05.
RESULTS
Pathway-Specific Targeting of 5-HT6 Receptors in NAcSh
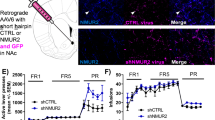
To increase expression of 5-HT6 receptors in either the direct or indirect pathway neurons selectively, we used HSV vectors that have been described (Figure 1a) (Eskenazi et al, 2015a; Ferguson et al, 2011, 2013; Michaelides et al, 2013). These vectors utilize the rat prodynorphin (Dyn) or the rat proenkephalin (Enk) promoter to induce transgene expression of either HA-tagged 5-HT6 receptors or GFP in dMSNs and iMSNs, respectively. The dMSN and iMSN manipulations were performed on separate cohorts of animals run at different times and were hence analyzed separately. The medial NAcSh was targeted, and accurate injections were confirmed histologically (Figure 1b); five animals were excluded due to inaccurate injections. Transgene expression using these Enk and Dyn promoters has been shown by our laboratory to be present up to 2 months post injection (Ferguson et al, 2008), and was confirmed in NAcSh 40 days post injection using immunohistochemistry (Figure 1c). The experimental timeline is illustrated in Figure 1d.
Pathway-specific targeting of 5-HT6 receptors in NAcSh. (a) Viral vector plasmid maps: plasmid maps for both experimental vectors expressing hemagglutinin (HA)-tagged 5-HT6 receptors via either the pENK promoter (Enk-5-HT6) or the pDYN promoter (Dyn-5-HT6). (b) Diagram depicting locations of viral injections in NAcSh in experimental animals. Hits are shown in green and misses are shown as empty circles. Inset dashed square shows area imaged for c. (c) Photomicrograph at × 5 magnification depicting the immunostaining of cells in the NAcSh infected with experimental virus Enk-5-HT6 more than 40 days past infection. Anti-HA antibody is shown in green. Inset shows same area at × 20 magnification. (d) Timeline of experimental procedure.
Increasing 5-HT6 Receptors in NAcSh iMSNs But Not in the dMSNs Decreased Operant Cocaine SA on an FR1 Schedule
In rats treated with Dyn-GFP or Dyn-5-HT6, there was no significant difference between viral vector treatment groups on the number of cocaine infusions (Figure 2a, F(1,13)=0.87, p=0.37), indicating that increased expression of 5-HT6 receptors in dMSNs did not alter cocaine reinforcement at a moderate unit dosage (0.75 mg/kg/infusion). The rats had established stable patterns of cocaine self-administration after the first 8 days; as a result, we examined the final 3 days of FR1 responding but there was no significant difference in average cocaine infusions between these treatment groups (Figure 2b, t-test, p=0.87). In contrast, in rats expressing either Enk-5-HT6 or Enk-GFP in medial NAcSh there was a significant difference between viral vector treatment groups on the number of cocaine infusions (Figure 2c, F(1,24)=5.32, p=0.03), indicating that increased expression of 5-HT6 receptors in iMSNs decreased cocaine SA on an FR1 reinforcement schedule. We examined the final 3 days of FR1 responding and the Enk-5-HT6-expressing rats took significantly less cocaine (Figure 2d, t-test, p=0.015). There was a significant difference between active and inactive lever presses in both the 5-HT6 receptor and GFP groups in the dMSNs (Supplementary Figure 1a, one-way ANOVA, p<0.001), as well as the 5-HT6 receptor and GFP in the iMSNs (Supplementary Figure 1b, one-way ANOVA, p<0.001) across all days of FR1 training.
Increasing 5-HT6 receptors in NAcSh iMSNs but not in the dMSNs decreased operant cocaine SA on an FR1 schedule. (a) Daily FR1 cocaine infusions (mean±SEM) for Dyn-GFP (n=7) and Dyn-5-HT6 (n=8) (two-way ANOVA, F(1,13)=0.87, p=0.37). (b) Average FR1 cocaine infusions during the last 3 days of training (mean±SEM) for Dyn-GFP (n=7) and Dyn-5-HT6 (n=8; t-test, p=0.87). (c) Daily FR1 cocaine infusions (mean±SEM) for Enk-GFP (n=12) and Enk-5-HT6 (n=14) (two-way ANOVA, F(1,24)=5.32; p=0.03). (d) Average FR1 cocaine infusions during the last 3 days of training (mean±SEM) for Enk-GFP (n=12) and Enk-5-HT6 (n=14) (t-test, p=0.015). *p<0.05.
Increased Expression of 5-HT6 Receptors in iMSNs Shifted the Dose-Preference Curve Downward
Rats were exposed to two additional doses of cocaine (0.375 and 1.5 mg/kg/infusion) for 3 days at each dose, starting with the low dose and followed by the high dose. We also included SA data from the last three training days at the 0.75-mg/kg/infusion dose in our analyses to depict the complete dose-preference curve. All groups had decreasing preference for the highest dose of cocaine as indicated by fewer infusions taken as dose increased. Rats with increased 5-HT6 receptors in the dMSNs did not differ from their GFP counterparts with regard to either the number of cocaine infusions (Figure 3a, F(1,29)=0.77, p=0.39) or the total cocaine taken (Figure 3b, F(1,29)=1.75, p=0.20). However, rats with increased 5-HT6 receptors in iMSNs administered significantly fewer cocaine infusions (Figure 3c, F(1,52)=16.85, p=0.0001) at the lower two doses as well as less cocaine across all doses (Figure 3d, F(1,52)=14.9, p=0.0003).
Increasing expression of 5-HT6 receptors in iMSNs shifted the dose-preference curve downward. (a) Dose-dependent cocaine infusion (mean±SEM) for rats with Dyn-GFP or Dyn-5-HT6 (Dyn-5-HT6, n=4–8 depending on dose; Dyn-GFP n=4–7 depending on dose) at three unit doses of cocaine infusion (0.375, 0.75, and 1.5 mg/kg/infusion) (two-way ANOVA, F(1,29)=0.77, p=0.39). (b) Total cocaine received (mean±SEM) for rats with Dyn-GFP or Dyn-5-HT6 at the three cocaine doses (0.375, 0.75, and 1.5 mg/kg) (two-way ANOVA, F(1,29)=1.75, p=0.20). (c) Dose-dependent cocaine infusion (mean±SEM) for rats with Enk-GFP or Enk-5-HT6 (Enk-5-HT6 n=8–13 depending on dose; Enk-GFP n=8–13 depending on dose) at the three doses (0.375, 0.75, and 1.5 mg/kg) (two-way ANOVA, F(1,52)=16.85; p=0.0001). (d) Total cocaine received (mean±SEM) for rats with Enk-GFP or Enk-5-HT6 at the three doses (0.375, 0.75, and 1.5 mg/kg) (two-way ANOVA, F(1,52)=14.9, p=0.0003). *p<0.05, **p<0.001, and ***p<0.0001.
Increasing Expression of 5-HT6 Receptors in the NAcSh iMSNs Changes Patterns of Cocaine Taking
To assess whether increased 5-HT6 receptors in either dMSNs or iMSNs affect the pattern of cocaine taking, we examined the time course (15 min intervals) of FR1 operant responding for a unit dose of 0.75 mg/kg/infusion. The number of infusions per 15-min bin over the course of the session did not change for rats with increased 5-HT6 receptors in dMSNs (Figure 4a, F(1,11)=2.56, p=0.14). However, the average number of infusions per 15-min bin over the course of the session was significantly decreased in rats with increased 5-HT6 receptors in iMSNs (Figure 4b, F(1,24)=9.07, p=0.006). We also examined the interval from the initiation of the session until the first response within each session. We found no change in the average time to first response for the group with increased 5-HT6 receptors in dMSNs (Figure 4c, t-test, p=0.63), but increased 5-HT6 receptors in iMSNs significantly increased the interval before the initial lever press of each session (Figure 4d, t-test, p=0.035).
Increasing expression of 5-HT6 receptor in the NAcSh iMSN changes patterns of cocaine taking and reduced the preferred brain cocaine concentration. (a) Average cocaine infusions (mean±SEM) per 15-min bin on mid-dose testing days shown for rats expressing either Dyn-GFP (n=7) or Dyn-5-HT6 (n=8) (two-way ANOVA, F(1,11)=2.56; p=0.14). (b) Average cocaine infusions (mean±SEM) per 15-min bin on mid-dose testing days shown for rats expressing either Enk-GFP (n=13) or Enk-5-HT6 (n=13) (two-way ANOVA, F(1,24)=9.07, p=0.006). (c) Average time to initial response in minutes for Dyn-GFP (n=7) and Dyn-5-HT6 (n=8) rats on all testing days (t-test, p=0.63). (d) Average time to initial response in minutes for Enk-GFP (n=13) and Enk-5-HT6 (n=14) rats on all testing days (t-test, p=0.035). Example brain cocaine concentration modeling at 0.75 mg/kg/infusion is shown for reference Enk-GFP (e) and Enk-5-HT6 (f). (g) Average estimated brain cocaine concentrations across doses (0.375, 0.75, and 1.5 mg/kg/infusion) for Dyn-5-HT6 rats (n=4–8 depending on dose) and Dyn-GFP rats (n=4–7 depending on dose) (two-way ANOVA, F(1,29)=1.94, p=0.18). (h) Average estimated brain cocaine concentrations across doses (0.375, 0.75, and 1.5 mg/kg/infusion) for Enk-5-HT6 rats (n=8–14 depending on dose) and Enk-GFP rats (n=8–14 depending on dose) (two-way ANOVA, F(1,54)=7.427, p=0.009). *p<0.05, **p<0.001, ***p<0.0001.
Increasing Expression of 5-HT6 Receptors in iMSNs Reduced the Preferred Brain Cocaine Concentration
To further probe the pattern of cocaine taking among rats, we used a previously described pharmacokinetic model to estimate local brain concentration of cocaine in NAc (Pan et al, 1991; Zimmer et al, 2011). For reference, examples of how the model predicted brain cocaine concentration during the SA session are shown for Enk-GFP and Enk-5-HT6 (Figure 4e and f, respectively). Estimated tissue cocaine concentrations were not different between Dyn-5-HT6 and Dyn-GFP rats at any unit dose tested (Figure 4g, F(1,29)=1.94, p=0.18). However, rats with increased 5-HT6 receptors in iMSNs titrated around significantly lower cocaine concentrations as compared with their GFP counterparts (Figure 4h, F(1,54)=7.427, p=0.0086).
Increasing Expression of 5-HT6 Receptors in Either the NAcSh dMSNs or iMSNs Did Not Affect Cocaine SA on PR Reinforcement Schedule
On a PR reinforcement schedule, increased 5-HT6 receptor expression in dMSNs had no significant effect on the total number of cocaine infusions when averaged over 4 PR testing days (Figure 5a, t-test, p=0.14) or average active lever presses (Figure 5b, t-test, p=0.264). Similarly, increased 5-HT6 receptor expression in iMSNs had no significant effect on the average number of cocaine infusions over 4 PR testing days (Figure 5c, t-test, p=0.31) or active lever presses (Figure 5d, t-test, p=0.53). Thus, we conclude that the motivation to self-administer cocaine at the dose of 0.75 mg/kg/infusion was not directly affected by 5-HT6 receptors in either pathway.
Increasing expression of 5-HT6 receptors in either the NAcSh dMSNs or the iMSNs does not affect operant responding on a PR schedule. (a) Cocaine infusions (mean±SEM) for the average of four PR sessions for Dyn-GFP (n=7) and Dyn-5-HT6 (n=7) (t-test, p=0.14). (b) Active lever presses (mean±SEM) for the average of four PR sessions for Dyn-GFP (n=7) and Dyn-5-HT6 (n=7) (t-test, p=0.26). (c) Cocaine infusions (mean±SEM) for the average of four PR sessions for Enk-GFP (n=12) and Enk-5-HT6 (n=14) (t-test, p=0.31). (d) Active lever presses (mean±SEM) for the average of four PR sessions for Enk-GFP (n=12) and Enk-5-HT6 (n=14) (t-test, p=0.53). (e) Increasing expression of 5-HT6 receptors the iMSNs increases preference for cocaine at a low dose. CPP score (cocaine time−saline time (s)) shown for rats with either Enk-GFP (n=8) or Enk-5-HT6 (n=8) (t-test, p=0.04). *p<0.05.
Increasing Expression of 5-HT6 Receptors in NAcSh iMSNs Increased CPP for Cocaine at a Low Dose
To examine whether increased 5-HT6 receptors in the indirect pathway altered the sensitivity to the rewarding properties of cocaine, we tested the effects of expressing 5-HT6 receptors or GFP in iMSNs on the ability of cocaine to support CPP to a low dose of cocaine (5 mg/kg) that we previously found was too low to produce a consistent CPP in control rats (Barot et al, 2007; Neumaier et al, 2002). The rats with increased 5-HT6 receptor expression in iMSNs spent a significantly longer period exploring the cocaine paired chamber during testing (Figure 5e, t-test, p=0.04).
Increasing Expression of 5-HT6 Receptors in dMSNs or iMSNs Does Not Influence Locomotor Activity
We tested whether increasing 5-HT6 receptors in dMSNs or iMSNs altered spontaneous locomotor activity in a subset of rats from all groups. All of the groups demonstrated similar locomotor activity patterns with no significant between-group differences (direct pathway: Supplementary Figure 2a, F(1,5)=0.30, p=0.61; indirect pathway: Supplementary Figure 2b, F(1,10)=2.42, p=0.151).
DISCUSSION
The segregation of dMSNs and iMSNs in dorsal and ventral striatum is a fundamental feature of the organization of brain reward circuitry, and the functional implications of the divergent roles of these pathways are currently a topic of great interest. It has been known for many years that both iMSNs and dMSNs express 5-HT6 receptors (Ward et al, 1995), but past studies involving 5-HT6 receptors and psychostimulants did not address these distinct pathways (de Bruin et al, 2013; Eskenazi and Neumaier, 2011a; van Gaalen et al, 2010; Valentini et al, 2012), and hence the circuitry mechanisms involved were not evaluated. Therefore, we investigated the relative contribution of 5-HT6 receptors in each pathway on operant behaviors reinforced by cocaine.
Previously, we found that increasing 5-HT6 receptors in both pathways of NAcSh interfered with learning of a CPP for cocaine, and that systemic treatment with a selective 5-HT6 antagonist had the opposite effect (Ferguson et al, 2008). However, in that study we did not observe any direct effects on drug reward. Furthermore, we have previously observed that increasing 5-HT6 receptors in both pathways of dorsomedial striatum interfered with acquisition of action-outcome learning when sucrose was used as the reinforcer (Eskenazi and Neumaier, 2011a; Mitchell et al, 2007). In dorsomedial striatum, we recently found that selective expression of 5-HT6 receptors in iMSNs alone was sufficient to interfere with action-outcome operant learning (Eskenazi et al, 2015a). In contrast, we found that 5-HT6 receptor signaling in dorsolateral striatum facilitated omission training in a contingency-specific manner, but did not affect the learning acquisition of action-outcome learning (Eskenazi and Neumaier, 2011b). Thus, the impact of 5-HT6 receptors on striatum-dependent behavior depends both on the pathway being manipulated and on the subregion that is targeted.
The central finding of this report is that increased expression of 5-HT6 receptors in the iMSNs reduced cocaine SA under an FR1 reinforcement schedule by about 50% when using a 0.75-mg/kg unit dose. The most obvious explanation for this result is that the animals had lower motivation to take cocaine, but increasing 5-HT6 receptors in iMSNs had no effect on the ‘break point’ under a PR reinforcement schedule at a dose of 0.75 mg/kg, suggesting that their motivation to take cocaine was not likely altered at this dose. In contrast, increasing expression of 5-HT6 receptors in the dMSNs of NAcSh had no effect on cocaine SA under either FR1 or PR reinforcement schedules. Although the control rats in the dMSN experiments self-administered slightly more cocaine than the rats in the control iMSN experiments, these cohorts were run at entirely separate times and the difference can be attributed to biological variation. Although it is possible that increasing 5-HT6 receptor expression may modulate break point at higher or lower doses of cocaine, the present evidence suggests that there were no changes in motivation using the PR ‘break point’ method.
One possible explanation for our central observation is that the rats were more sensitive to the reinforcing properties of cocaine and therefore required less to attain a preferred subjective response to the drug. We applied a previously developed and later refined method for modeling the pharmacokinetics and tissue concentration of cocaine to estimate the brain cocaine concentration that each animal titrated to and presumably preferred for each unit dose of cocaine tested (Pan et al, 1991; Zimmer et al, 2011). This analysis suggested that the Enk-5-HT6 rats preferred a lower tissue concentration of cocaine, further supporting the notion that they are more sensitive to the reinforcing properties of cocaine.
We further tested this idea using a different behavioral model by performing a cocaine CPP experiment using conditions that enhance the sensitivity to detect differences in reward as compared with aversion—ie, low dose and brief pairing of cocaine with the context (Barot et al, 2007; Ettenberg, 2004; Pliakas et al, 2001). The rats with increased 5-HT6 receptors in iMSNs developed a stronger preference for a typically subthreshold dose of cocaine (5 mg/kg i.p.) (Barot et al, 2007; Neumaier et al, 2002), suggesting that these rats were more sensitive to the rewarding effects of cocaine at this dose.
We considered alternative interpretations as well. It is also conceivable that increasing 5-HT6 receptors in the iMSNs altered the processing of aversive information. However, we found no differences in operant self-administration at the highest cocaine dose (1.5 mg/kg unit dose), which is most likely to generate aversive effects. We did not test place conditioning with high dose cocaine and delayed pairing, which is a sensitive method for detecting aversion (Barot et al, 2007; Ettenberg, 2004; Pliakas et al, 2001) because there were no differences in cocaine SA at the highest unit dose tested. Further, we found no differences in operant responding on a PR reinforcement schedule. Taken together, this suggests that these results are not likely due to changes in the processing of aversive information. Another explanation is that increased 5-HT6 receptors in NAcSh altered motor activity and interfered with cocaine taking, but there were no changes in locomotion following these manipulations nor were there differences at 1.5 mg/kg cocaine, a unit dose that would be most likely to induce motor deficits. In a previous study we also found no evidence that increased 5-HT6 receptors altered the acute locomotor response to cocaine or sensitization over several days, although in that study we expressed 5-HT6 receptors using a pathway non-selective viral vector (Ferguson et al, 2008).
Although we observed no change in PR responding at 0.75 mg/kg, which is usually interpreted to mean that there was no change in motivation to take cocaine at this dose, Enk-5-HT6 animals displayed a consistent pattern of delaying the initial cocaine infusions during each test session, across multiple days. It is unlikely that 5-HT6 receptors impaired the animal’s association between cocaine availability and the active lever, because there was no declination in the delay to initial lever press over multiple days. However, most of the differences between Enk-GFP and Enk-5-HT6 treated rats occurred during the beginning of each session, as the experimental animals lacked the initial burst of cocaine taking that is typical of cocaine SA (Belin et al, 2009; Zimmer et al, 2011). The higher initial rate of cocaine infusions may be taken to attain a preferred cocaine level in the brain, followed by a second phase of slower cocaine taking to maintain a preferred cocaine concentration in the brain. If this interpretation is correct, then 5-HT6 receptors in the NAcSh indirect pathway have an impact on the initial phase of cocaine SA and it is not surprising that there was no change in break point under a PR schedule, which depends on an extended cocaine SA session. In a recent study, we found that increased 5-HT6 receptors in iMSNs in dorsomedial striatum delayed the time to initial lever press only on the first day of training for sucrose responding (Eskenazi et al, 2015a), which is a similar result but this effect was not sustained over multiple days as it was in the present study using cocaine SA. If 5-HT6 receptors in iMSNs of the NAcSh produce increased sensitivity to lower doses of cocaine, then this might in turn reduce the likelihood of developing compulsive addiction-like behaviors which are associated with higher infusion rates of cocaine SA during early experience with drug taking (Belin et al, 2009, 2011). This interpretation is supported by previous work showing that activating the indirect pathway fosters resilience to compulsive cocaine use (Bock et al, 2013) and reducing indirect pathway activity can facilitate psychomotor sensitization to cocaine (Ferguson et al, 2011).
One parsimonious way to interpret all of this data together is to consider the distribution of 5-HT6 receptors in both of these output pathways. Although dopamine differentially activates dMSNs via D1 receptors and inhibits iMSNs via D2 receptors, serotonin will excite both pathways as both populations of MSNs express endogenous 5-HT6 receptors (Ward et al, 1995).Thus, dMSNs are activated by both the endogenous Gαs-coupled D1 and 5-HT6 receptors, leading to an accumulation of cAMP (Dobi et al, 2011; Surmeier et al, 2007). However, D2 and 5-HT6 receptors in iMSNs have opposite effects on adenylate cyclase activity and will tend to oppose one another. Therefore, 5-HT6 receptors in the iMSNs are positioned to interfere with behaviors that are supported by dopamine action in NAcSh. Presumably balanced activation of endogenous 5-HT6 receptors in both pathways simultaneously interferes with dopamine actions by reducing the differential activation of these two pathways. It is not known whether exposure to abused drugs alters the expression levels of 5-HT6 in these pathways differentially, but this is a topic under active exploration.
The finding that increased 5-HT6 receptor expression in the iMSNs of NAcSh leads to an increase in the sensitivity to the reinforcing properties of cocaine while maintaining normal reward motivation properties has implications toward the field of drug addiction research. Although similar studies have focused on the role of 5-HT6 receptors modulating DA in NAcSh (Valentini et al, 2012) during cocaine reinforcement, or the role of the indirect pathway in reducing cocaine reinforcement (Hikida et al, 2010), no studies have focused on the pathway-specific roles for 5-HT6 receptors. Our findings suggest that 5-HT6 receptors are a potential target for treatment of drug addiction, in that if receptors are selectively upregulated or stimulated in the iMSNs, the amount of drug taken by individuals might reduce, thereby impeding the progression toward compulsive, unregulated drug use.
Funding and disclosure
The authors declare no conflict of interest.
References
Andrews C, Lucki I (2001). Effects of cocaine on extracellular dopamine and serotonin levels in the nucleus accumbens. Psychopharmacology (Berl) 155: 221–229.
Barot SK, Ferguson SM, Neumaier JF (2007). 5-HT1B receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci 25: 3125–3131.
Belin D, Balado E, Piazza PV, Deroche-Gamonet V (2009). Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry 65: 863–868.
Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V (2011). High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36: 569–579.
Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF et al (2013). Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16: 632–638.
Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM et al (2015). Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci 35: 7927–7937.
de Bruin NMWJ, McCreary AC, van Loevezijn A, de Vries TJ, Venhorst J, van Drimmelen M et al (2013). A novel highly selective 5-HT6 receptor antagonist attenuates ethanol and nicotine seeking but does not affect inhibitory response control in Wistar rats. Behav Brain Res 236: 157–165.
Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA (2011). Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci 31: 1895–1904.
Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501: 179–184.
Eskenazi D, Brodsky M, Neumaier JF (2015a). Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience 299: 97–106.
Eskenazi D, Brodsky M, Neumaier JF (2015b). Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience 299: 97–106.
Eskenazi D, Neumaier JF (2011a). Increased expression of the 5-HT6 receptor by viral mediated gene transfer into posterior but not anterior dorsomedial striatum interferes with acquisition of a discrete action-outcome task. J Psychopharmacol 25: 944–951.
Eskenazi D, Neumaier JF (2011b). Increased expression of 5-HT6 receptors in dorsolateral striatum decreases habitual lever pressing, but does not affect learning acquisition of simple operant tasks in rats. Eur J Neurosci 34: 343–351.
Ettenberg A (2004). Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev 27: 721–728.
Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y et al (2011). Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14: 22–24.
Ferguson SM, Mitchell ES, Neumaier JF (2008). Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry 63: 207–213.
Ferguson SM, Phillips PEM, Roth BL, Wess J, Neumaier JF (2013). Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci 33: 11668–11676.
Fijal K, Pachuta A, Mccreary AC, Nowak E, Papp M, Bieñkowski P et al (2010). Effects of serotonin (5-HT) 6 receptor ligands on responding for cocaine reward and seeking in rats. Pharmacol Reports 181187: 1005–1014.
Frantz KJ, Hansson KJ, Stouffer DG, Parsons LH (2002). 5-HT 6 receptor antagonism potentiates the behavioral and neurochemical effects of amphetamine but not cocaine. Neuropharmacology 42: 170–180.
Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ et al (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432.
Gerfen CR, McGinty JF, Young WS (1991). Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci 11: 1016–1031.
Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66: 896–907.
Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW et al (2003). Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 64: 1295–1308.
Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598.
Macpherson T, Morita M, Hikida T (2014). Striatal direct and indirect pathways control decision-making behavior. Front Psychol 5: 1–7.
McDevitt Ra, Tiran-Cappello A, Shen H, Balderas I, Britt JP, Marino RAM et al (2014). Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep 8: 1857–1869.
Michaelides M, Anderson SAR, Ananth M, Smirnov D, Thanos PK, Neumaier JF et al (2013). Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest 123: 5342–5350.
Mitchell ES, Neumaier JF (2008). 5-HT6 receptor antagonist reversal of emotional learning and prepulse inhibition deficits induced by apomorphine or scopolamine. Pharmacol Biochem Behav 88: 291–298.
Mitchell ES, Sexton T, Neumaier JF (2007). Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology 32: 1520–1530.
Müller CP, Homberg JR (2015). The role of serotonin in drug use and addiction. Behav Brain Res 277: 146–192.
Nair SG, Furay AR, Liu Y, Neumaier JF (2013). Differential effect of viral overexpression of nucleus accumbens shell 5-HT1B receptors on stress- and cocaine priming-induced reinstatement of cocaine seeking. Pharmacol Biochem Behav 112: 89–95.
Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA (2002). Elevated expression of 5-HT 1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci 22: 10856–10863.
Pan H-T, Menacherry S, Justice JB (1991). Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 56: 1299–1306.
Parsons LH, Justice JB (1993a). Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res 606: 195–199.
Parsons LH, Justice JB (1993b). Serotonin and dopamine sensitization in the nucleus accumbens, ventral tegmental area, and dorsal raphe nucleus following repeated cocaine administration. J Neurochem 61: 1611–1619.
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403.
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J (2009). Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373: 2223–2233.
Richardson NR, Roberts DCS (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11.
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB et al (2001). Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA 98: 5300–5305.
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S et al (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475: 377–380.
Surmeier DJ, Ding J, Day M, Wang Z, Shen W (2007). D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235.
Tassone A, Madeo G, Schirinzi T, Vita D, Puglisi F, Ponterio G et al (2011). Activation of 5-HT6 receptors inhibits corticostriatal glutamatergic transmission. Neuropharmacology 61: 632–637.
Tzschentke TM (1998). Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56: 613–672.
US Department of Justice National Drug Intelligence Center (2011). National Drug Threat Assessment 2011. 1–72 doi:doi:10.1037/e618352012-001.
Uchimura N, North R (1990). Actions of cocaine on rat nucleus accumbens neurones in vitro. Br J Pharmacol 740: 736–740.
United States Department of Health and Human Services (2014). The Health Consequences of Smoking—50 years of progress: a report of the Surgeon General. Rep Surg Gen 1081.
Valentini V, Piras G, De Luca MA, Perra V, Bordi F, Borsini F et al (2012). Evidence for a role of a dopamine/5-HT6 receptor interaction in cocaine reinforcement. Neuropharmacology 65C: 58–64.
van Gaalen MM, Schetters D, Schoffelmeer ANM, De Vries TJ (2010). 5-HT6 antagonism attenuates cue-induced relapse to cocaine seeking without affecting cocaine reinforcement. Int J Neuropsychopharmacol 13: 961–965.
Ward R, Hamblin M, Lachowicz J, Hoffman BJ, Sibley DR, Dorsa DM (1995). Localization of serotonin subtype 6 receptor messenger RNA in the rat brain by in situ hybridization histochemistry. Neuroscience 64: 1105–1111.
Yager LM, Garcia AF, Wunsch AM, Ferguson SM (2015). The ins and outs of the striatum: role in drug addiction. Neuroscience 301: 529–541.
Zimmer BA, Dobrin CV, Roberts DCS (2011). Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology 36: 2741–2749.
Acknowledgements
Support for this work was funded by NIDA Training Grant T32-DA00007278 (MB), R01DA030807 (JFN) and R21DA034192 (SGN). We gratefully acknowledge the technical support offered by Michele Kelly, Scott Ng-Evans, and Adam Lesiak. We thank David Roberts for providing the software program to estimate tissue cocaine concentrations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Brodsky, M., Gibson, A., Smirnov, D. et al. Striatal 5-HT6 Receptors Regulate Cocaine Reinforcement in a Pathway-Selective Manner. Neuropsychopharmacol 41, 2377–2387 (2016). https://doi.org/10.1038/npp.2016.45
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.45