Abstract
Impairments in inhibitory control and in stimulus-driven attention are hallmarks of drug addiction and are associated with decreased activation in the right inferior frontal gyrus (IFG). Although previous studies indicate that the response inhibition function is impaired in abstinent heroin dependents, and that this is mediated by reduced IFG activity, it remains completely unknown whether and how an acute dose of heroin modulates IFG activity during cognitive control in heroin-dependent patients. This study investigates the acute effects of heroin administration on IFG activity during response inhibition and stimulus-driven attention in heroin-dependent patients. Using a cross-over, double-blind, placebo-controlled design, saline and heroin were administered to 26 heroin-dependent patients from stable heroin-assisted treatment, while performing a Go/No–Go event-related functional magnetic resonance imaging task to assess right IFG activity during motor response inhibition, as well as during oddball-driven attention allocation. Relative to saline, heroin significantly reduced right IFG activity during both successful response inhibition and oddball-driven attention allocation, whereas it did not change right IFG activity during response inhibition after correction for the effect of attention allocation. These heroin-induced effects were not related to changes in drug craving, state anxiety, behavioral performance, or co-consumption of psychostimulant drugs. This study demonstrates that heroin administration acutely impairs stimulus-driven attention allocation, as indicated by reduced IFG activity in response to infrequently presented stimuli, and does not specifically modulate IFG activity during response inhibition.
Similar content being viewed by others
INTRODUCTION
Drug addiction is recognized as a severe relapsing brain disorder (Leshner, 1997), characterized by impaired cognitive self-control, including compromised ability to exert control over drug urges or to inhibit impulsive drug-driven behavior (Perry and Carroll, 2008). This cognitive loss of inhibitory control in addiction was initially proposed to be derived from dopamine-mediated neuronal circuits involved in reward learning, conditioning, and habit formation (Everitt et al, 2001). However, several functional magnetic resonance imaging (fMRI) studies have demonstrated the crucial contribution of the prefrontal cortex (PFC), especially the right inferior frontal gyrus (IFG), to inhibitory control (Egner, 2011). For example, response inhibition—as often operationalized by the Go/No–Go task—has been associated with significant activation of the right IFG in healthy volunteers (Simmonds et al, 2008), while dysfunctional activity in the right IFG during response inhibition has repeatedly been reported as a prominent hallmark in drug addiction (Goldstein and Volkow, 2011).
It has been shown that frontal lobe-mediated executive functioning, such as impulse control, is impaired in heroin-dependent subjects (Pau et al, 2002). The impaired inhibitory control in abstinent heroin-addicted individuals is accompanied by reduced right IFG activity during the Go/No–Go task (Fu et al, 2008). Furthermore, the right IFG is activated not only during response inhibition but also during stimulus-driven attention allocation (Chikazoe et al, 2009). It has been shown that heroin abuse is also associated with deficits in attentional set-shifting (Ornstein et al, 2000) and that opiate-dependent participants actively enrolled in a methadone-maintaining program showed reduced selective attention (Bracken et al, 2012). A recent study explored the cognitive deficits in heroin abusers and showed that the decreased ability to shift attention and inhibit inappropriate response tendencies, which cumulate with years of consumption, may be mediated by dysfunctional IFG activity (Lundqvist, 2010). In keeping with this, the severity of heroin consumption is negatively correlated with brain metabolism in the right IFG (Moreno-López et al, 2012).
Intravenously injected heroin is rapidly converted to 6-monoacetylmorphine and morphine. Heroin mainly exerts its effects through μ and κ opiate receptor agonism; the μ opiate receptor subtype is critical for the rewarding effects of heroin and morphine, because blockade of μ opiate receptors, but not of other receptors, attenuates opiate self-administration (De Vries and Shippenberg, 2002). The PFC has a high density of opiate receptors (Wager et al, 2007) and has been implicated in the mediation of abnormal goal-directed behavior in drug addiction (Koob and Volkow, 2010). Although heroin-assisted treatment, including the prescription of pharmaceutical heroin (diacetylmorphine), has consistently been found to be an effective treatment for severe heroin addiction (Haasen et al, 2007), the acute effects of heroin administration during response inhibition and stimulus-driven attention allocation in heroin-maintained patients remain completely unknown.
In this study, we have examined whether and how heroin administration acutely modulates neural activity during response inhibition and stimulus-driven attention allocation in heroin-dependent patients, relative to a saline injection. Using an event-related Go/No–Go fMRI paradigm, which has been shown to be sensitive to pharmacological challenges (Borgwardt et al, 2008; Rubia et al, 2005), we specifically focused on the right IFG as a region of interest. A second reason for the selection of the right IFG was that previous fMRI studies in cocaine-dependent patients had revealed a direct relationship between right IFG activity and behavioral performance during the Go/No–Go task after an intravenous dose of cocaine (Garavan et al, 2008; Li et al, 2010). Given that heroin addiction is associated with impairments in both impulse control and selective attention (Bracken et al, 2012; Pau et al, 2002), we hypothesized that heroin would acutely reduce the right IFG activity during both of these processes.
MATERIALS AND METHODS
Patients
The study was approved by the local ethics committee and registered under http://clinicaltrials.gov (ID NCT01174927). After receiving a written and oral description of the study aims, all participants gave their written informed consent before inclusion.
Twenty-six heroin-maintained outpatients (19 male and 7 female subjects, mean age 41.1±6.8) were recruited from the Centre of Substance Use Disorders of the Department of Psychiatry in Basel University. The inclusion criteria were as follows: age older than 18 years and a past history of intravenous heroin use with current heroin-maintained treatment for at least 6 months, with an unchanged heroin dose during the past 3 months. The exclusion criteria were a positive alcohol breathalyzer test, or an additional physical disease or psychiatric disorder, including other comorbid conditions such as substance dependencies. Clinically experienced psychiatrists conducted the Structured Clinical Interview for DSM-IV Axis II Disorders (15) to assess the diagnosis of comorbid personality disorders.
When heroin-dependent patients fulfilled the inclusion criteria, the history of use of heroin and other illicit substances was assessed with a semi-structured interview according to the ICD-10 research criteria. Subjects reported their years of education (mean=10.23±2.54 years), smoking behavior (cigarettes per day: mean=21.46±9.19), age of first heroin use (mean=18.88±3.46 years), years of dependence (mean=20.54±6.56 years), and daily heroin dose (326±130.97 mg).
Patients were told to abstain from drug consumption other than the prescribed heroin administration for the duration of the study, as well as to abstain from alcohol intake and smoking 72 and 2 h before scanning, respectively. Nevertheless, 8 patients were tested positive for cannabis and 12 patients for cocaine at one or both points of the measurement.
Drug Administration
Saline and heroin were administered through an indwelling intravenous catheter over a period of 30 s, using a cross-over, double-blind, vehicle-controlled design. Heroin hydrochloride (Swiss Federal Office of Public Health) was dissolved on site in 5 ml of sterile water and aspirated into a syringe, following the procedure described by Stohler et al (1999). Subjects who received their individualized dose of heroin before the first scanning session received 5 ml of saline before the second session, and vice versa. Furthermore, on both sessions all subjects received both heroin and saline. That is, the subjects who received heroin before scanning were administered vehicle after scanning (ie, 60 min after the first injection), whereas the subjects who received saline before scanning were administered heroin after scanning.
Psychometric Assessments
Patients’ drug craving (‘desire to use heroin’) was assessed before and 60 min after saline/heroin injection, using the 45-item Heroin Craving Questionnaire (Tiffany, 1999), which measures positive and negative aspects of craving on five theory-derived nine-item scales. The German version of the State-Trait Anxiety Inventory was used to quantify state anxiety before and after both treatment conditions (Spielberger et al, 1970).
fMRI Go/No–Go Paradigm
Thirty minutes after drug administration, all patients underwent an event-related Go/No–Go fMRI paradigm that was conducted with jittered inter-stimulus intervals (ISIs) and incorporated infrequently presented oddball stimuli to optimize statistical efficiency. The task is a well-validated paradigm, requiring either the execution or the inhibition of a motor response, depending on the visual presentation of the stimuli (Rubia et al, 2006). The basic Go task is a choice reaction time paradigm, in which arrows point either to the left or to the right side for 500 ms, with a mean ISI of 1800 ms (jitter range: 1600–2000 ms). During Go trials, subjects were instructed to press a left or right response button according to the direction of the arrow. In 11% of the trials, arrows pointing upward appeared. During these so-called ‘No–Go’ trials, participants were required to inhibit their motor response. During another 11% of the trials, arrows pointing left or right at a 23° angle were presented, and subjects were told to respond to these in the same way as for Go stimuli (even though they pointed obliquely). These ‘oddball’ stimuli were used to control for novelty effects associated with the low frequency and different orientation of the No–Go relative to the Go trials (stimulus-driven attention allocation). In total, there were 24 No–Go, 160 Go, and 24 oddball trials, with task duration of approximately 6 min.
fMRI Image Acquisition and Analysis
Scanning was performed on a 3T scanner (Siemens Magnetom Verio; Siemens Healthcare, Erlangen, Germany), using an echo planar sequence with 2.5 s repetition time, 28 ms echo time, a matrix size of 76 × 76, and 38 slices with 0.5-mm interslice gap, providing a resolution of 3 × 3 × 3 mm3 and a field of view of 228 × 228 cm2. In total, 160 volumes were acquired.
Analysis of fMRI data was conducted with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). All volumes were realigned to the first volume, normalized into a standard stereotactic space (Montreal Neurological Institute (MNI)), and smoothed using a 8-mm full-width at half-maximum Gaussian kernel. During model specification, onset times for correctly given responses during Go, No–Go, and oddball trials were convolved with a canonical hemodynamic response function. We also modeled time and dispersion derivatives, while the motion parameters acquired during the realignment procedure were added to the individual design matrix as multiple regressors.
Subject-specific condition effects during response inhibition (‘No–Go vs Go trials’) and oddball-driven attention allocation (‘oddball vs Go trials’) were computed using t-contrast, producing a contrast image propagated to the second-level analysis. To extract response inhibition more precisely, so that oddball-driven attention allocation is excluded, a contrast of ‘No–Go vs oddball trials’ was also computed.
One-sample t-tests for both treatment conditions were performed to investigate the effects of response inhibition and oddball-driven attention allocation, whereas we used a paired t-test to compare heroin and saline treatments. To investigate unpredicted regions of activation, we conducted a whole-brain analysis. Small volume correction (sphere of 5 mm radius as recently performed (Hayama et al, 2012)) was used for clusters observed in the a priori hypothesized region of interest (ie, right IFG) (Worsley et al, 1996). The MNI coordinates of x=50, y=16, and z=22, as taken from the Automated Talairach Atlas (Tzourio-Mazoyer et al, 2002), served as the anatomical reference point. As some patients revealed no activated voxels at these coordinates, small volume correction was performed at the nearest suprathreshold voxels. The statistical threshold was adjusted to provide a family-wise error (FWE) of P<0.05 at peak and cluster level (based on the spatial extent of clusters of voxels at the threshold of P<0.01), corrected for multiple comparisons across the whole brain or the a priori hypothesized region of interest. As this study was intended to look at acute heroin effects on the right IFG, we additionally employed a second analysis, in which an anatomical mask only consisting of the right IFG was applied explicitly to the second-level analyses, providing a closer look into the region of interest.
Behavioral Task Performance and Symptom-Rating Analyses
Behavioral task performance was evaluated on the one hand by the probability of inhibition and on the other hand by the sensitivity index d′ and response bias c, using the formula d′=z(Hits)−z(FA) and c=−0.5*(z(FA)+z(Hits)), where FA reflects false alarms. Differences between the two treatment conditions were examined using paired t-tests. State anxiety and craving ratings were entered into two repeated measures analysis of variance (ANOVA) with within-subjects factors, treatment (saline vs heroin), and condition (pre vs post treatment).
Results
Behavioral Task Performance and Symptom Rating
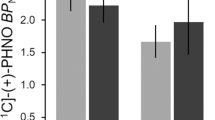
Repeated measures ANOVA on the state-anxiety scores revealed a significant treatment × condition interaction (F(1,25)=42.11; P<0.0001). Bonferroni post-hoc testing further showed that state-anxiety scores were significantly reduced by heroin (P<0.05), but not saline. Furthermore, a treatment × condition interaction (F(1,25)=75.34; P<0.0001) indicated that saline and heroin differentially affected patients’ drug craving. Post analysis revealed that craving ratings significantly increased after the administration of saline compared with the pretreatment condition (P<0.05), whereas heroin administration significantly decreased craving scores (P<0.0001) (Figure 1).
Patients’ drug craving as expressed by the ‘desire to use heroin’ pre- and post-treatment. Note: significant differences between pre- and post-treatment at *P<0.05 and **P<0.0001.
No significant differences in the probability of inhibition were found between heroin (mean% (SD)=92.31% (16.77)) and saline (mean% (SD)=91.99% (15.71)) (T=−0.71; P=0.94). Furthermore, no significant differences between saline and heroin administration were found with respect to the sensitivity index d′ (T=0.40; P=0.69) and the response bias c (T=−1.56; P=0.13).
Right IFG Activity During Response Inhibition and Oddball-Driven Attention Allocation
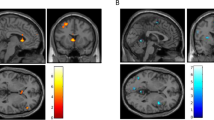
For the ‘No–Go vs Go’ contrast, no activation survived correction for multiple comparisons across the whole brain after the administration of saline. Significant activation was found in the lingual and superior temporal gyrus after heroin exposure. After adjusting for SVC, right IFG activation was significant in both treatment conditions. For the ‘No–Go vs oddball’ contrast, significant activation was found in the postcentral gyrus and superior frontal gyrus in the saline condition, but no significant activation was found in the heroin condition after correction for multiple comparisons. The right IFG was significant in both conditions after adjusting with SVC. For the ‘oddball vs Go’ contrast, no significant activations were found for the two conditions across the whole brain after correction for multiple comparisons, while the activity in the right IFG was significant after adjusting for SVC correction (Table 1, Figure 2). The results of the within-mask analysis are provided in the Supplementary information (Supplementary Table S1; Supplementary Figure S1).
Brain activation during the Go/No–Go task for response inhibition (‘No–Go vs Go’ and ‘No–Go vs oddball’) and oddball-driven attention allocation (‘oddball vs Go’) after saline and heroin administration in heroin-dependent patients. The right IFG activity survived small volume correction for multiple comparisons (FWE-corrected at peak and cluster level at P<0.05). Note: (1) ‘No–Go vs Go trials’, (2) ‘No–Go vs oddball trials’, and (3) ‘oddball vs Go trials’.
Acute Heroin Effect on Right IFG Activity
No heroin-induced difference in activation survived correction for multiple comparisons across the whole brain, but after employing SVC, right IFG activity was significantly reduced at the peak and cluster levels (FWE-corrected at P<0.05) (Table 2, Figure 3). The results of the within-mask analysis are provided in the Supplementary information (Supplementary Table S2, Supplementary Figure S2).
Significant differences in right IFG activity (blue crosshairs) between the saline and heroin conditions (saline>heroin) (small volume corrected at peak and cluster level, FWE-corrected at P<0.05). Note: we found no significant voxels that were FWE cluster-level corrected for multiple comparisons across the whole brain at P<0.05.
A multivariate GLM with heroin-induced changes on right IFG activity as dependent variables, cocaine and cannabis consumption as fixed factors, and changes in drug craving, state anxiety, behavioral performance, and nicotine consumption as covariates found no significant relationships (Supplementary Table S3).
DISCUSSION
In the present study, we explored whether and how right IFG activity in heroin-dependent patients was modulated after acute heroin administration during cognitive control. Specifically, we examined whether heroin acutely modulated right IFG activity during successful response inhibition and attention allocation to infrequently presented oddball stimuli in long-lasting heroin-maintained dependents, relative to a saline injection. Two major results were found: First, heroin acutely reduced the activity in the right IFG during both successful response inhibition (‘No–Go vs Go’ contrast) and oddball-driven attention allocation (‘oddball vs Go’ contrast). Second, a more specific index of response inhibition (‘No–Go vs oddball’ contrast) was not modulated by heroin.
In accordance with previous fMRI studies demonstrating the crucial role of the right IFG during response inhibition and oddball-driven attention allocation (Chikazoe et al, 2009; Rubia et al, 2006), we found right IFG activation following heroin and saline administration during both of these cognitive processes. The direct associations between the right IFG and inhibitory performance are supported by consistent evidence from fMRI lesions (Aron et al, 2004) and TMS studies (Jacobson et al, 2011). By further comparing the IFG activation after the saline and heroin administration, our analysis revealed that heroin acutely reduced right IFG activity during both successful response inhibition and oddball-driven attention allocation. In contrast, no significant differences in the behavioral task performances were found between heroin and saline administration. However, the current study was specifically designed to detect acute drug effects on brain activation, rather than on behavioral performance. Significant effect on brain activations, but not task performance, is a common finding in fMRI studies and has previously been shown in other Go/No–Go studies after pharmacological interventions (Borgwardt et al, 2008; Rubia et al, 2005). This can be explained by the fact that functional neuroimaging techniques detect changes at the physiological level and are more sensitive than behavioral measures (Wilkinson and Halligan, 2004). Thus, our findings suggest that heroin acutely impairs motor response inhibition and stimulus-driven attention allocation in heroin-maintained patients, as reflected in the reduced BOLD signal within the right IFG, but not in behavioral performance, as the paradigm used was underpowered to find behavioral differences. This fits with previous results, showing that the behavioral deficits during the performance of the Go/No–Go task in heroin-addicted individuals are associated with a reduced BOLD response in the IFG (Fu et al, 2008) and that the severity of heroin consumption was negatively correlated with brain metabolism in the right IFG (Moreno-López et al, 2012). No significant relationship between the acute heroin effect on right IFG activity and craving was found; this was consistent with previous evidence demonstrating that craving in heroin dependence is more related to activation in mesolimbic brain areas (Li et al, 2012).
However, as the No–Go trials in the conducted paradigm can also be understood as oddball trials, ie, as infrequently presented stimuli embedded in stream of continuous Go trials, it is possible that the No–Go stimulus may also recruit cognitive control processes other than response inhibition. There is evidence that the IFG is also activated in response to unexpected infrequent stimuli, compared with expected frequently presented stimuli (Doricchi et al, 2010). Such infrequently presented oddball stimuli represent novelty-alerting signals that help to interrupt endogenous-oriented attention (Corbetta et al, 2008). This is consistent with a previous study indicating that the inferior frontal junction has no role in response inhibition (Aron and Poldrack, 2006), and suggesting that the inferior frontal junction is also involved in cognitive processes other than response inhibition. This means that the contrast of ‘No–Go vs Go trials’ does not clearly dissociate activation associated with response inhibition and oddball-driven attention allocation. To extract response inhibition more precisely and in such a way that attentional processes can be excluded, we calculated a more specific index of response inhibition by contrasting ‘No–Go vs oddball trials’. We found that heroin did not modulate IFG activity relative to saline in this more specific response inhibition contrast. Thus, our findings indicate that heroin acutely affects the IFG during oddball-driven attention allocation rather than having a specific effect on response inhibition. This corresponds with previous evidence that heroin abuse is associated with deficits in attentional set-shifting (Ornstein et al, 2000) and that opiate-dependent participants actively enrolled in a methadone-maintaining program showed reduced selective attention (Bracken et al, 2012).
In contrast to our findings of reduced right IFG activity after acute heroin administration to heroin-dependent subjects without any change in behavioral performance, intravenous administration of cocaine to cocaine users acutely improves inhibitory control, accompanied by increased activation in the right dorsolateral PFC and right insula, extending into right IFG, during the Go/No–Go task (Garavan et al, 2008). Administration of methylphenidate consistently improved cognitive control in cocaine-dependent patients, while this effect positively correlated with frontal cortex activation (Li et al, 2010). Thus, a key finding of the present study is that acute heroin administration produces very different effects from the stimulant cocaine. This discrepancy is further reflected in the comparison with healthy subjects during the Go/No–Go task. Although right IFG activity in heroin-dependent subjects is significantly reduced (Fu et al, 2008), no such difference from healthy controls was found in chronic cocaine users (Kaufman et al, 2003). These findings are reminiscent of a recent review reporting distinct behavioral and neurobiological differences between opiate and psychostimulant addiction during prefrontal-dependent cognitive functioning, in particular those functions related to impulsivity (Badiani et al, 2011). At the psychological level, heroin and cocaine differ in terms of factors that induce craving and in terms of their use in daily life, while environmental factors also interact with the drug effects (Badiani et al, 2011). In particular, heroin consumption is preferred in the home environment, but cocaine outside the home (Caprioli et al, 2009).
Our functional results on the right IFG activity are in line with volumetric abnormalities of this brain region. In healthy subjects, performance levels on inhibitory control tasks were correlated with gray matter in the IFG (Tabibnia et al, 2011). Compared with healthy subjects, the deficient behavioral performance in methamphetamine-dependent subjects was related to lower gray matter volume in the IFG, suggesting that cognitive control involves a common substrate in the IFG and that successful cognitive performance depends on the integrity of this substrate (Tabibnia et al, 2011). It has been shown that heroin-dependent subjects also had reduced GMV in the right PFC (Liu et al, 2009). Remarkably, in long-term heroin-dependent individuals, the duration of heroin use correlated negatively with the density of GM in the right IFG (Yuan et al, 2010; Yuan et al, 2009) and positively with reductions in right frontal white matter (Bora et al, 2012), suggesting that the duration of heroin use is a critical factor leading to frontal brain damage. Relative to cocaine-induced changes on GMV, the structural alterations in the PFC are less pronounced in heroin addicts (Verdejo-García et al, 2007), further indicating that the effects of heroin and cocaine consumption are distinct. Furthermore, significant reductions in GMV of the right IFG have also been reported in heroin-dependent individuals on methadone-maintenance treatment (Lin et al, 2012; Lyoo et al, 2006). Based on these findings, we may speculate that our fMRI findings are partly mediated by structural alterations in the IFG as a result of long-term heroin use.
With respect to the underlying neuropharmacological mechanisms, drug addiction is associated with neuroplastic alterations, mainly in the dopaminergic system, but with interactive effects on the glutamatergic neurotransmission. As recently proposed (Koob and Volkow, 2010), the transition to addiction involves adaptations in a broad neuronal circuitry, which may begin with changes in the mesolimbic dopamine system, with a subsequent cascade into the prefrontal brain regions, such as the IFG or cingulate gyrus, leading to aberrant prefrontal–striatal connections. This circuitry has a high density of μ opiate receptors (Wager et al, 2007), which are crucial in mediating the effects of intravenous opiate self-administration, but have only a minor part in psychostimulant self-administration. Despite fundamental differences at the neuropharmacological level, but in line with cocaine-dependent subjects, heroin dependence is also associated with low D2 receptor binding and low presynaptic dopamine in the striatum. But in contrast to cocaine addiction, these parameters of striatal dopamine transmission were not correlated with the choice to self-administer the drug, further reflecting distinct differences between heroin and cocaine dependence (Martinez et al, 2012). Furthermore, repeated heroin use induces adaptations in glutamatergic neurotransmission (Noda and Nabeshima, 2004), and a recent study in opiate-dependent patients during opiate-maintenance therapy indicated destabilization of the glutamate system (Hermann et al, 2012). Although the neuropharmacological mechanisms of acute heroin administration in heroin-maintained patients are not well understood, we may speculate that the heroin-induced alteration of right IFG activity is associated with alterations in the glutamatergic and dopaminergic systems.
This study focused on right IFG activity and did not take into account functionally related cortical and subcortical brain regions. However, the neuronal network engaged in response inhibition and stimulus-driven attention is complex and, apart from the IFG, comprises several other regions, such as the anterior cingulate cortex, orbitofrontal cortices, superior parietal cortex, insula, thalamus, and cerebellum. For example, recent evidence showed functional connectivity during response inhibition between the right IFG and other brain regions in the frontal, striatal, and parietal cortices (Duann et al, 2009), while the processing of oddball stimuli not only activated the right PFC but also the tempo–parietal junction (Rubia et al, 2010). Thus, our right IFG findings might derive from abnormalities in functional connectivity to other brain regions implicated in cognitive control. Such analysis might also help to bring more insights into the neuronal differences between heroin and psychostimulant addiction. We intend to address this problem in future studies by using model-based techniques of effective connectivity. Furthermore, changes in right IFG activity did not survive correction for multiple comparisons across the whole brain when the two treatment conditions were treated separately. This can partly be explained by the fact that our population consisted of patients showing significantly reduced activity in the right IFG during the Go/No–Go task compared with healthy subjects (Fu et al, 2008; Lee et al, 2005). Although we could increase the degree of freedom by pooling the two treatment conditions to obtain more robust activations, we specifically aimed to explore how heroin acutely modulates right IFG activity in a task that has repeatedly been shown to induce robust IFG activity (Borgwardt et al, 2008; Rubia et al, 2006). Finally, anatomically different parts within the IFG may differentially contribute to response inhibition and stimulus-driven attention (Chikazoe et al, 2009). This should be addressed in future studies.
In conclusion, to the best of our knowledge, this is the first fMRI study examining the neuronal effects of acute heroin administration on response inhibition and oddball-driven attention allocation in heroin-dependent patients actively enrolled in a long-lasting heroin-maintenance therapy. This study demonstrates that heroin administration acutely impairs stimulus-driven attention allocation, as indicated by the reduced IFG activity in response to infrequently presented stimuli, and does not specifically modulate IFG activity during response inhibition. Finally, our findings further underpin the dissociable behavioral and neuronal effects of chronic heroin and cocaine use on IFG-dependent cognitive control, reflecting the importance of studying drug addiction by comparing several classes of addictive drugs.
FUNDING AND DISCLOSURE
AR-R has received research grants from the European Network of National Schizophrenia Networks Studying Gene-Environment Interactions (EU-FP7); the Foundation Alamaya for neurobiological research in schizophrenia; Österreichische Forschungsförderungsgesellschaft mbH, Vienna, Austria; the Swiss National Science Foundation for different project; and the Stanley Medical Research Foundation, USA, for the NEURAPRO (North America, EURope, Australia PROdrome) Study. Unconditional research grants were received from Servier Suisse S.A., Orpha Swiss GmbH, Eli Lilly, Pfizer, and Mepha Pharma. The remaining authors declare no conflict of interest. The work was not supported by pharmaceutical industry grants.
References
Aron AR, Monsell S, Sahakian BJ, Robbins TW (2004). A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain 127 (Pt 7): 1561–1573.
Aron AR, Poldrack RA (2006). Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26: 2424–2433.
Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12: 685–700.
Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L et al (2012). White matter microstructure in opiate addiction. Addict Biol 17: 141–148.
Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML et al (2008). Neural basis of delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry 64: 966–973.
Bracken BK, Trksak GH, Penetar DM, Tartarini WL, Maywalt MA, Dorsey CM et al (2012). Response inhibition and psychomotor speed during methadone maintenance: impact of treatment duration, dose, and sleep deprivation. Drug Alcohol Depend 125: 132–139.
Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A (2009). Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biol Psychiatry 65: 893–899.
Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S et al (2009). Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex 19: 146–152.
Corbetta M, Patel G, Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324.
De Vries TJ, Shippenberg TS (2002). Neural systems underlying opiate addiction. J Neurosci 22: 3321–3325.
Doricchi F, Macci E, Silvetti M, Macaluso E (2010). Neural correlates of the spatial and expectancy components of endogenous and stimulus-driven orienting of attention in the Posner task. Cereb Cortex 20: 1574–1585.
Duann JR, Ide JS, Luo X, Li CS (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci 29: 10171–10179.
Egner T (2011). Right ventrolateral prefrontal cortex mediates individual differences in conflict-driven cognitive control. J Cogn Neurosci 23: 3903–3913.
Everitt BJ, Dickinson A, Robbins TW (2001). The neuropsychological basis of addictive behavior. Brain Res Brain Res Rev 36: 129–138.
Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L et al (2008). Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett 438: 322–326.
Garavan H, Kaufman JN, Hester R (2008). Acute effects of cocaine on the neurobiology of cognitive control. Philos Trans R Soc Lond B Biol Sci 363: 3267–3276.
Goldstein RZ, Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669.
Haasen C, Verthein U, Degkwitz P, Berger J, Krausz M, Naber D (2007). Heroin-assisted treatment for opioid dependence: randomised controlled trial. Br J Psychiatry 191: 55–62.
Hayama HR, Vilberg KL, Rugg MD (2012). Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? J Cogn Neurosci 24: 1127–1137.
Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstädt-Klein S et al (2012). MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol 17: 659–667.
Jacobson L, Javitt DC, Lavidor M (2011). Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci 23: 3380–3387.
Kaufman JN, Ross TJ, Stein EA, Garavan H (2003). Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23: 7839–7843.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC (2005). Neural activity associated with cognitive regulation in heroin users: a fMRI study. Neurosci Lett 382: 211–216.
Leshner AI (1997). Addiction is a brain disease, and it matters. Science 278: 45–47.
Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL et al (2010). Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci USA 107: 14455–14459.
Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J et al (2012). Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res 1469: 63–72.
Lin WC, Chou KH, Chen HL, Huang CC, Lu CH, Li SH et al (2012). Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry Res 201: 89–97.
Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, Xu L et al (2009). Frontal and cingulate gray matter volume reduction in heroin dependence: optimized voxel-based morphometry. Psychiatry Clin Neurosci 63: 563–568.
Lundqvist T (2010). Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci 3: 247–275.
Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J et al (2006). Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl) 184: 139–144.
Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A et al (2012). Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 71: 192–198.
Moreno-López L, Stamatakis EA, Fernández-Serrano MJ, Gómez-Río M, Rodríguez-Fernández A, Pérez-García M et al (2012). Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: a resting-PET brain metabolism study. PLoS One 7: e39830.
Noda Y, Nabeshima T (2004). Opiate physical dependence and N-methyl-D-aspartate receptors. Eur J Pharmacol 500: 121–128.
Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ et al (2000). Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23: 113–126.
Pau CW, Lee TM, Chan SF (2002). The impact of heroin on frontal executive functions. Arch Clin Neuropsychol 17: 663–670.
Perry JL, Carroll ME (2008). The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 200: 1–26.
Rubia K, Hyde Z, Halari R, Giampietro V, Smith A (2010). Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage 51: 817–827.
Rubia K, Lee F, Cleare AJ, Tunstall N, Fu CH, Brammer M et al (2005). Tryptophan depletion reduces right inferior prefrontal activation during response inhibition in fast, event-related fMRI. Psychopharmacology (Berl) 179: 791–803.
Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E et al (2006). Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp 27: 973–993.
Simmonds DJ, Pekar JJ, Mostofsky SH (2008). Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46: 224–232.
Spielberger C, Gorsuch R, Lusheme R (1970) STAI, Manual for the State-Trait-Anxiety-Inventory. Consulting Psychologists Press: Palo Alto.
Stohler R, Dürsteler KM, Störmer R, Seifritz E, Hug I, Sattler-Mayr J et al (1999). Rapid cortical hemoglobin deoxygenation after heroin and methadone injection in humans: a preliminary report. Drug Alcohol Depend 57: 23–28.
Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S et al (2011). Different forms of self-control share a neurocognitive substrate. J Neurosci 31: 4805–4810.
Tiffany ST (1999). Cognitive concepts of craving. Alcohol Res Health 23: 215–224.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289.
Verdejo-García A, Pérez-García M, Sánchez-Barrera M, Rodriguez-Fernández A, Gómez-Río M (2007). [Neuroimaging and drug addiction: neuroanatomical correlates of cocaine, opiates, cannabis and ecstasy abuse]. Rev Neurol 44: 432–439.
Wager TD, Scott DJ, Zubieta JK (2007). Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA 104: 11056–11061.
Wilkinson D, Halligan P (2004). The relevance of behavioral measures for functional-imaging studies of cognition. Nat Rev Neurosci 5: 67–73.
Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73.
Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P et al (2010). Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neurosci Lett 482: 101–105.
Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y et al (2009). Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn 71: 223–228.
Acknowledgements
We would like to acknowledge the infrastructural support of the Medical Image Analysis Centre, University Hospital Basel. We would also like to thank Mrs Brown for language editing. This study was funded by the Swiss National Science Foundation (SNSF) (32003B-127544) (MW, SB, GW, and AR-R) and FAG Basel (MW).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Schmidt, A., Walter, M., Gerber, H. et al. Inferior Frontal Cortex Modulation with an Acute Dose of Heroin During Cognitive Control. Neuropsychopharmacol 38, 2231–2239 (2013). https://doi.org/10.1038/npp.2013.123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.123
Keywords
This article is cited by
-
Do mindfulness-based interventions change brain function in people with substance dependence? A systematic review of the fMRI evidence
BMC Psychiatry (2023)
-
Impaired belief updating and devaluation in adult women with bulimia nervosa
Translational Psychiatry (2023)
-
Changes in cognitive and behavioral control after lamotrigine and intensive dialectical behavioral therapy for severe, multi-impulsive bulimia nervosa: an fMRI case study
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Suicide Risk and Addiction: The Impact of Alcohol and Opioid Use Disorders
Current Addiction Reports (2021)
-
Mesolimbic dopamine D2 receptors and neural representations of subjective value
Scientific Reports (2019)