Abstract
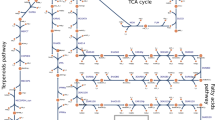
The cyanobacterial tricarboxylic acid (TCA) cycle functions in both in biosynthesis and energy generation. However, it has until recently been generally considered to be incomplete1,2 with limited flux3,4, and few attempts have been made to draw carbon from the cycle for biotechnological purposes. We demonstrated that ethylene can be sustainably and efficiently produced from the TCA cycle of the recombinant cyanobacterium Synechocystis 6803 expressing the Pseudomonas ethylene-forming enzyme (Efe)5. A new strain with a modified ribosome binding site upstream of the efe gene diverts 10% of fixed carbon to ethylene and shows increased photosynthetic activities. The highest specific ethylene production rate reached 718 ± 19 μl l–1 h–1 per A730 nm. Experimental and computational analyses based on kinetic 13C-isotope tracer and liquid chromatography coupled with mass spectrometry (LC–MS) demonstrated that the TCA metabolism is activated by the ethylene forming reaction, resulting in a predominantly cyclic architecture. The outcome significantly enhanced flux through the remodelled TCA cycle (37% of total fixed carbon) compared with a complete, but bifurcated and low-flux (13% of total fixed carbon) TCA cycle in the wild type. Global carbon flux is redirected towards the engineered ethylene pathway. The remarkable metabolic network plasticity of this cyanobacterium is manifested by the enhancement of photosynthetic activity and redistribution of carbon flux, enabling efficient ethylene production from the TCA cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pearce, J., Leach, C. K. & Carr, N. G. The incomplete tricarboxylic acid cycle in the blue-green alga Anabaena variabilis. J. Gen. Microbiol. 55, 371–378 (1969).
Smith, A. J., London, J. & Stanier, R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J. Bacteriol. 94, 972–983 (1967).
Young, J. D., Shastri, A. A., Stephanopoulos, G. & Morgan, J. A. Mapping photoautotrophic metabolism with isotopically nonstationary 13C flux analysis. Metab. Eng. 14, 185–185 (2012).
You, L., Berla, B., He, L., Pakrasi, B. H. & Tang, Y. J. 13C-MFA delineates the photomixotrophic metabolism of Synechocystis sp. PCC 6803 under light- and carbon-sufficient conditions. Biotechnol. J. 9, 684–692 (2014).
Ungerer, J. et al. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energ. Environ. Sci. 5, 8998–9006 (2012).
Sheehan, J. Engineering direct conversion of CO2 to biofuel. Nature Biotechnol. 27, 1128–1129 (2009).
Ducat, D. C., Way, J. C. & Silver, P. A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 29, 95–103 (2011).
Stephanopoulos, G. & Vallino, J. J. Network rigidity and metabolic engineering in metabolite overproduction. Science 252, 1675–1681 (1991).
Melis, A. Carbon partitioning in photosynthesis. Curr. Opin. Chem. Biol. 17, 453–456 (2013).
Davies, F. K., Work, V. H., Beliaev, A. S. & Posewitz, M. C. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2, 21 (2014).
Guerrero, F., Carbonell, V., Cossu, M., Correddu, D. & Jones, P. R. Ethylene synthesis and regulated expression of recombinant protein in Synechocystis sp PCC 6803. PLoS ONE 7, e50470 (2012).
Zhu, T., Xie, X. M., Li, Z. M., Tan, X. M. & Lu, X. F. Enhancing photosynthetic production of ethylene in genetically engineered Synechocystis sp PCC 6803. Green Chem. 17, 421–434 (2015).
Jazmin, L. J. & Young, J. D. Isotopically nonstationary 13C metabolic flux analysis. Syst. Metab. Eng. Methods Mol. Biol. 985, 367–390 (2013).
Cooley, J. W., Howitt, C. A. & Vermaas, W. F. J. Succinate: quinol oxidoreductases in the cyanobacterium Synechocystis sp strain PCC 6803: presence and function in metabolism and electron transport. J. Bacteriol. 182, 714–722 (2000).
Cooley, J. W. & Vermaas, W. F. J. Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp strain PCC 6803: capacity comparisons and physiological function. J. Bacteriol. 183, 4251–4258 (2001).
Fukuda, H. et al. Two reactions are simultaneously catalyzed by a single enzyme: the arginine-dependent simultaneous formation of two products, ethylene and succinate, from 2-oxoglutarate by an enzyme from Pseudomonas syringae. Biochem. Biophys. Res. Commun. 188, 483–489 (1992).
Labarre, J., Thuriaux, P. & Chauvat, F. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp strain 6803. J. Bacteriol. 169, 4668–4673 (1987).
Quintero, M. J., Montesinos, M. L., Herrero, A. & Flores, E. Identification of genes encoding amino acid permeases by inactivation of selected ORFs from the Synechocystis genomic sequence. Genome Res. 11, 2034–2040 (2001).
Young, J. D. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 30, 1333–1335 (2014).
Ducat, D. C., Avelar-Rivas, J. A., Way, J. C. & Silver, P. A. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microb. 78, 2660–2668 (2012).
Liu, X. Y., Sheng, J. & Curtiss, R. Fatty acid production in genetically modified cyanobacteria. Proc. Natl Acad. Sci. USA 108, 6899–6904 (2011).
Deng, M. D. & Coleman, J. R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microb. 65, 523–528 (1999).
Lan, E. I. & Liao, J. C. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc. Natl Acad. Sci. USA 109, 6018–6023 (2012).
Angermayr, S. A., Paszota, M. & Hellingwerf, K. J. Engineering a cyanobacterial cell factory for production of lactic acid. Appl. Environ. Microb. 78, 7098–7106 (2012).
Sweetlove, L. J., Beard, K. F. M., Nunes-Nesi, A., Fernie, A. R. & Ratcliffe, R. G. Not just a circle: flux modes in the plant TCA cycle. Trends Plant Sci. 15, 462–470 (2010).
Zhang, S. Y. & Bryant, D. A. The tricarboxylic acid cycle in cyanobacteria. Science 334, 1551–1553 (2011).
Xiong, W., Brune, D. & Vermaas, W. F. J. The gamma-aminobutyric acid shunt contributes to closing the tricarboxylic acid cycle in Synechocystis sp PCC 6803. Mol. Microbiol. 93, 786–796 (2014).
Lopez-Bucio, J., Nieto-Jacobo, M. F., Ramirez-Rodriguez, V. & Herrera-Estrella, L. Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 160, 1–13 (2000).
Nunes-Nesi, A. et al. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 137, 611–622 (2005).
Nunes-Nesi, A., Araujo, W. L. & Fernie, A. R. Targeting mitochondrial metabolism and machinery as a means to enhance photosynthesis. Plant Physiol. 155, 101–107 (2011).
Acknowledgements
This work is supported by the National Renewable Energy Laboratory Director's Fellowship (W.X.), and the DOE Energy Efficiency and Renewable Energy (EERE) BioEnergy Technologies Office (J.Y., B.W.), EERE Fuel Cell Technologies Office (P.C.M.), and DOE Office of Science BER grant DE-SC0008628 (J.A.M.). We are grateful to Jamey D. Young of Vanderbilt University for providing software and technical assistance on 13C metabolic modelling, and to Maria Ghirardi, Carrie Eckert and William Michener for helpful discussion or assistance with LC–MS equipment.
Author information
Authors and Affiliations
Contributions
W.X., J.A.M., P.C.M. and J.Y. conceived the idea, and edited the manuscript. J.U. constructed the strains. B.W. analysed the Efe protein levels. W.X. designed and performed the experiments, analysed data and wrote most of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Xiong, W., Morgan, J., Ungerer, J. et al. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene. Nature Plants 1, 15053 (2015). https://doi.org/10.1038/nplants.2015.53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nplants.2015.53
This article is cited by
-
Divide and conquer towards synthetic autotrophy
Nature Catalysis (2023)
-
Artificial photosynthetic cells with biotic–abiotic hybrid energy modules for customized CO2 conversion
Nature Communications (2023)
-
Optimal energy and redox metabolism in the cyanobacterium Synechocystis sp. PCC 6803
npj Systems Biology and Applications (2023)
-
A guanidine-degrading enzyme controls genomic stability of ethylene-producing cyanobacteria
Nature Communications (2021)
-
Metabolic engineering of a fast-growing cyanobacterium Synechococcus elongatus PCC 11801 for photoautotrophic production of succinic acid
Biotechnology for Biofuels (2020)