Abstract
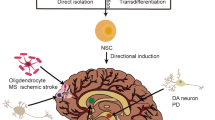
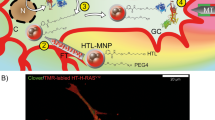
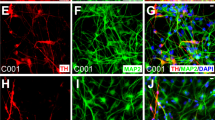
Electromagnetic fields (EMF) are physical energy fields generated by electrically charged objects, and specific ranges of EMF can influence numerous biological processes, which include the control of cell fate and plasticity. In this study, we show that electromagnetized gold nanoparticles (AuNPs) in the presence of specific EMF conditions facilitate an efficient direct lineage reprogramming to induced dopamine neurons in vitro and in vivo. Remarkably, electromagnetic stimulation leads to a specific activation of the histone acetyltransferase Brd2, which results in histone H3K27 acetylation and a robust activation of neuron-specific genes. In vivo dopaminergic neuron reprogramming by EMF stimulation of AuNPs efficiently and non-invasively alleviated symptoms in mouse Parkinson's disease models. This study provides a proof of principle for EMF-based in vivo lineage conversion as a potentially viable and safe therapeutic strategy for the treatment of neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Golbach, L. A. et al. Calcium homeostasis and low-frequency magnetic and electric field exposure: a systematic review and meta-analysis of in vitro studies. Environ. Int. 92-93, 695–706 (2016).
National Institute of Environmental Health Sciences and National Institutes of Health EMF Electric and Magnetic Fields Associated with the Use of Electric Power (NIEHS/DOE EMF Rapid Program, 2002).
Lester, G., Aaron, R. K., Neame, P. & Caterson, B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J. Orthop. Res. 20, 40–50 (2002).
De Mattei, M. et al. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect. Tissue Res. 44, 154–159 (2003).
Funk, R. H., Monsees, T. & Özkucur, N. Electromagnetic effects—from cell biology to medicine. Prog. Histochem. Cytochem. 43, 177–264 (2009).
Cho, H. et al. Neural stimulation on human bone marrow-derived mesenchymal stem cells by extremely low frequency electromagnetic fields. Biotechnol. Prog. 28, 1329–1335 (2012).
Seong, Y., Moon, J. & Kim, J. Egr1 mediated the neuronal differentiation induced by extremely low-frequency electromagnetic fields. Life Sci. 102, 16–27 (2014).
Motohashi, T. et al. Gene array analysis of neural crest cells identifies transcription factors necessary for direct conversion of embryonic fibroblasts into neural crest cells. Biol. Open 5, 311–322 (2016).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Marro, S. et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9, 374–382 (2011).
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Kim, J. et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 9, 413–419 (2011).
Torper, O. et al. Generation of induced neurons via direct conversion in vivo. Proc. Natl Acad. Sci. USA 110, 7038–7043 (2013).
Guo, Z. et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell 14, 188–202 (2014).
Salata, O. V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2, 3 (2004).
Morris, S. A. et al. Dissecting engineered cell types and enhancing cell fate conversion via CellNet. Cell 158, 889–902 (2014).
Chanda, S. et al. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Rep. 3, 282–296 (2014).
Hu, W. et al. Direct conversion of normal and Alzheimer's disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell 17, 204–212 (2015).
Li, X. et al. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell 17, 195–203 (2015).
Hei, W. H. et al. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics 37, 163–174 (2016).
LeRoy, G., Rickards, B. & Flint, S. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell 30, 51–60 (2008).
Torper, O. et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 12, 474–481.
Delle Monache, S., Alessandro, R., Iorio, R., Gualtieri, G. & Colonna, R. Extremely low frequency electromagnetic fields (ELF-EMFs) induce in vitro angiogenesis process in human endothelial cells. Bioelectromagnetics 29, 640–648 (2008).
Simko, M., Kriehuber, R., Weiss, D. & Luben, R. Effects of 50 Hz EMF exposure on micronucleus formation and apoptosis in transformed and nontransformed human cell lines. Bioelectromagnetics 19, 85–91 (1998).
Blank, M. & Goodman, R. Electromagnetic fields stress living cells. Pathophysiology 16, 71–78 (2009).
Baek, S. et al. Electromagnetic fields mediate efficient cell reprogramming into a pluripotent state. ACS Nano 8, 10125–10138 (2014).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012).
Rivetti di Val Cervo, P. et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson's disease model. Nat. Biotechnol. 35, 444–452 (2017).
Muraoka, N. & Ieda, M. Direct reprogramming of fibroblasts into myocytes to reverse fibrosis. Ann. Rev. Physiol. 76, 21–37 (2014).
Chen, J. X., Plonowska, K. & Wu, S. M. Somatic cell reprogramming into cardiovascular lineages. J. Cardiovasc. Pharmacol. Ther. 19, 340–349 (2014).
Hei, W. H. et al. Schwann-like cells differentiated from human dental pulp stem cells combined with a pulsed electromagnetic field can improve peripheral nerve regeneration. Bioelectromagnetics 37, 163–174 (2016).
Loginov, V. Accumulation of calcium ions in myocardial sarcoplasmic reticulum of restrained rats exposed to the pulsed electromagnetic field. Aviakosm. Ekolog. Med. 26, 49–51 (1991).
Bastus, N. G., Comenge, J. & Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: size focusing versus Ostwald ripening. Langmuir 27, 11098–11105 (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Ramírez, F., Dündar, F., Diehl, S., Grüning, B. A. & Manke, T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014).
Zhang, Y. et al. Model-based analysis of ChIP-seq (MACS). Genome Biol. 9, 1 (2008).
Yu, G., Wang, L.-G &, He Q.-Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Mootha, V. K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Cahan, P. et al. CellNet: network biology applied to stem cell engineering. Cell 158, 903–915 (2014).
Acknowledgements
This work was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2017M3A9C6029306, 2016R1A2B2014195, 2013M3A9B4076483, NRF-2015 M3A9B4051064), Korea Health Technology R&D Project, Ministry of Health & Welfare (HI16C1176), the Next-Generation BioGreen 21 Program, Rural Development Administration (PJ011077) and the Ministry of Food and Drug Safety in 2017 (14172MFDS974).
Author information
Authors and Affiliations
Contributions
J.Y., Y.K. and J.K. designed the study. J.Y., E.L., H.Y.K., D.Y., J.J., H.K., Y.C., W.L., J.S., S.B., W.Jang, W.Jun, S.K., J.H. and H.P. performed the experiments. J.Y., J.K. and C.J.L. analysed the data. Y.K., E.L., H.Y.K., D.Y. and S.H.M. contributed materials and/or analysis tools. The manuscript was written based on contributions by all the authors. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4409 kb)
Rights and permissions
About this article
Cite this article
Yoo, J., Lee, E., Kim, H. et al. Electromagnetized gold nanoparticles mediate direct lineage reprogramming into induced dopamine neurons in vivo for Parkinson's disease therapy. Nature Nanotech 12, 1006–1014 (2017). https://doi.org/10.1038/nnano.2017.133
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2017.133
This article is cited by
-
Progress in direct reprogramming of dopaminergic cell replacement therapy
Neurological Sciences (2024)
-
Identification of Parkinson’s disease-associated chromatin regulators
Scientific Reports (2023)
-
Efficient generation of brain organoids using magnetized gold nanoparticles
Scientific Reports (2023)
-
Low-frequency magnetic response of gold nanoparticles
Scientific Reports (2023)
-
Wireless charging-mediated angiogenesis and nerve repair by adaptable microporous hydrogels from conductive building blocks
Nature Communications (2022)