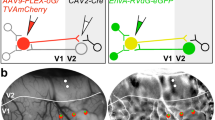
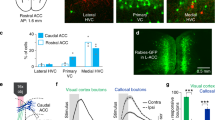
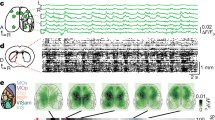
Abstract
Long-range projections from the frontal cortex are known to modulate sensory processing in multiple modalities. Although the mouse has become an increasingly important animal model for studying the circuit basis of behavior, the functional organization of its frontal cortical long-range connectivity remains poorly characterized. Here we used virus-assisted circuit mapping to identify the brain networks for top-down modulation of visual, somatosensory and auditory processing. The visual cortex is reciprocally connected to the anterior cingulate area, whereas the somatosensory and auditory cortices are connected to the primary and secondary motor cortices. Anterograde and retrograde tracing identified the cortical and subcortical structures belonging to each network. Furthermore, using new viral techniques to target subpopulations of frontal neurons projecting to the visual cortex versus the superior colliculus, we identified two distinct subnetworks within the visual network. These findings provide an anatomical foundation for understanding the brain mechanisms underlying top-down control of behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Xu, N.L. et al. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature 492, 247–251 (2012).
Lee, S., Kruglikov, I., Huang, Z.J., Fishell, G. & Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 16, 1662–1670 (2013).
Nelson, A. et al. A circuit for motor cortical modulation of auditory cortical activity. J. Neurosci. 33, 14342–14353 (2013).
Schneider, D.M., Nelson, A. & Mooney, R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014).
Zhang, S. et al. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science 345, 660–665 (2014).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Fuster, J.M. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe (Lippincott-Raven, Philadelphia, 1997).
Mao, T. et al. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123 (2011).
Noudoost, B., Chang, M.H., Steinmetz, N.A. & Moore, T. Top-down control of visual attention. Curr. Opin. Neurobiol. 20, 183–190 (2010).
Oh, S.W. et al. A mesoscale connectome of the mouse brain. Nature 508, 207–214 (2014).
Petersen, C.C. Cortical control of whisker movement. Annu. Rev. Neurosci. 37, 183–203 (2014).
Stanton, G.B., Bruce, C.J. & Goldberg, M.E. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J. Comp. Neurol. 353, 291–305 (1995).
Zingg, B. et al. Neural networks of the mouse neocortex. Cell 156, 1096–1111 (2014).
Hooks, B.M. et al. Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J. Neurosci. 33, 748–760 (2013).
Hunnicutt, B.J. et al. A comprehensive thalamocortical projection map at the mesoscopic level. Nat. Neurosci. 17, 1276–1285 (2014).
Miyamichi, K. et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature 472, 191–196 (2011).
Wickersham, I.R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Wang, Q., Sporns, O. & Burkhalter, A. Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. J. Neurosci. 32, 4386–4399 (2012).
Jones, E.G. The Thalamus 2nd edn. (Cambridge University Press, 2007).
Wu, H., Williams, J. & Nathans, J. Complete morphologies of basal forebrain cholinergic neurons in the mouse. Elife 3, e02444 (2014).
Fishell, G. & Rudy, B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu. Rev. Neurosci. 34, 535–567 (2011).
Xu, X., Roby, K.D. & Callaway, E.M. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 518, 389–404 (2010).
Lodato, S., Shetty, A.S. & Arlotta, P. Cerebral cortex assembly: generating and reprogramming projection neuron diversity. Trends Neurosci. 38, 117–125 (2015).
Shepherd, G.M. Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013).
Makino, H. & Komiyama, T. Learning enhances the relative impact of top-down processing in the visual cortex. Nat. Neurosci. 18, 1116–1122 (2015).
Pouget, P. et al. Visual and motor connectivity and the distribution of calcium-binding proteins in macaque frontal eye field: implications for saccade target selection. Front. Neuroanat. 3, 2 (2009).
Goldberg, M.E. & Wurtz, R.H. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J. Neurophysiol. 35, 560–574 (1972).
Horwitz, G.D. & Newsome, W.T. Separate signals for target selection and movement specification in the superior colliculus. Science 284, 1158–1161 (1999).
Ignashchenkova, A., Dicke, P.W., Haarmeier, T. & Thier, P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat. Neurosci. 7, 56–64 (2004).
Kustov, A.A. & Robinson, D.L. Shared neural control of attentional shifts and eye movements. Nature 384, 74–77 (1996).
Zénon, A. & Krauzlis, R.J. Attention deficits without cortical neuronal deficits. Nature 489, 434–437 (2012).
Li, N., Chen, T.W., Guo, Z.V., Gerfen, C.R. & Svoboda, K. A motor cortex circuit for motor planning and movement. Nature 519, 51–56 (2015).
Beier, K.T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015).
Lerner, T.N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Euston, D.R., Gruber, A.J. & McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070 (2012).
Robinson, D.A. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 12, 1795–1808 (1972).
Wang, L., Liu, M., Segraves, M.A. & Cang, J. Visual experience is required for the development of eye movement maps in the mouse superior colliculus. J. Neurosci. 35, 12281–12286 (2015).
Fu, Y. et al. A cortical circuit for gain control by behavioral state. Cell 156, 1139–1152 (2014).
Goard, M. & Dan, Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 12, 1444–1449 (2009).
Metherate, R. & Ashe, J.H. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse 14, 132–143 (1993).
Pinto, L. et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863 (2013).
Nelson, A. & Mooney, R. The basal forebrain and motor cortex provide convergent yet distinct movement-related inputs to the auditory cortex. Neuron 90, 635–648 (2016).
Brown, S.P. & Hestrin, S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–1136 (2009).
Kiritani, T., Wickersham, I.R., Seung, H.S. & Shepherd, G.M. Hierarchical connectivity and connection-specific dynamics in the corticospinal-corticostriatal microcircuit in mouse motor cortex. J. Neurosci. 32, 4992–5001 (2012).
Morishima, M. & Kawaguchi, Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J. Neurosci. 26, 4394–4405 (2006).
Purushothaman, G., Marion, R., Li, K. & Casagrande, V.A. Gating and control of primary visual cortex by pulvinar. Nat. Neurosci. 15, 905–912 (2012).
Moore, T. & Armstrong, K.M. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421, 370–373 (2003).
Noudoost, B. & Moore, T. Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375 (2011).
Erlich, J.C., Bialek, M. & Brody, C.D. A cortical substrate for memory-guided orienting in the rat. Neuron 72, 330–343 (2011).
Maheshri, N., Koerber, J.T., Kaspar, B.K. & Schaffer, D.V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198–204 (2006).
Weissbourd, B. et al. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83, 645–662 (2014).
Lim, B.K., Huang, K.W., Grueter, B.A., Rothwell, P.E. & Malenka, R.C. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487, 183–189 (2012).
Wall, N.R., Wickersham, I.R., Cetin, A., De La Parra, M. & Callaway, E.M. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc. Natl. Acad. Sci. USA 107, 21848–21853 (2010).
Osakada, F. & Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601 (2013).
Acknowledgements
We thank B. Kim, S. Zhu and P. Kim for technical help. We thank L. Luo, K. Beier, B. Lim and E. Callaway for viral vectors and cell lines. We thank T. Kamigaki for discussion. This work was supported by NIH R01 EY018861.
Author information
Authors and Affiliations
Contributions
S.Z. and Y.D. conceived and designed the experiments. S.Z. performed and organized all the experiments. M.X. wrote the software for data analyses and analyzed the data. W.-C.C., C.M. and J.P.H.D. prepared AAV and RV vectors for rabies-virus-based retrograde tracing. W.-C.C. also performed some viral injections. T.L. and D.J. performed the detection for labeled cells and axons in some brain samples. J.L.F. and D.J. performed the brain tissue sectioning with a cryostat. S.Z., M.X. and Y.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Distribution of starter cells for RV-mediated retrograde tracing.
Parcellation of cortical areas is based on Allen Mouse Brain Atlas and drawn to scale. Thick white curves, outlines of VIS, SS, AUD, ACA and MO. Thin gray curves, outlines of primary visual cortex (VISp), barrel field of somatosensory cortex (SSbfd) and secondary motor cortex (MOs). Color scale, normalized starter cell density. Scale bar, 1 mm.
Supplementary Figure 3 Whole-brain distributions of inputs to VIS, SS and AUD.
(a) Viral vectors and injection procedure for RV-mediated trans-synaptic retrograde tracing. (b) Fluorescence images of LGd, VP and MG (yellow box in coronal diagram), showing RV-labeled input neurons (red) to VIS, SS and AUD, respectively (scale bar, 100 μm). Red, tdTomato; blue, DAPI. (c-e) RV-labeled neurons detected in all samples of each group (VIS, blue; SS, red; AUD, green; scale bar, 1 mm). White masks indicate injection sites excluded from data analysis. (f) Percentages of retrogradely labeled input neurons in selected cortical and subcortical brain regions (VIS, n = 3 mice; SS, n = 3; AUD, n = 3). Included are cortical areas with > 2% labeling and thalamic structures with > 1% labeling. Each circle represents one mouse. Error bar, ± s.e.m.
Supplementary Figure 5 Whole-brain distributions of axonal projections from VIS, SS and AUD.
(a) Viral vector and injection procedure for tracing axonal projections. (b) Fluorescence images of LGd/LGv, VP and MG (yellow box in coronal diagram), showing the axons (red) from VIS, SS and AUD, respectively (scale bar, 200 μm). Red, mCherry; blue, DAPI. (c-e) Axons detected in all samples of each group (VIS, blue; SS, red; AUD, green; scale bar, 1 mm). White masks indicate injection sites excluded from data analysis. (f) Percentages of labeled axons in cortical and subcortical brain structures (VIS, n = 3 mice; SS, n = 3; AUD, n = 3). Included are cortical areas with > 1% labeling and thalamic structures with > 0.8% labeling. Each circle represents one mouse. Error bar, ±s.e.m.
Supplementary Figure 6 Example experiments showing reciprocal connections between the frontal cortices and the sensory cortices.
(a) Fluorescence images of VIS, SS and AUD (red box in coronal diagram), showing the axons from ACA or MO (scale bar, 200 μm). Red, mCherry; blue, DAPI. (b) Right, fluorescence images of VIS, SS and AUD (red box in coronal diagram), showing RV-labeled input neurons (red) to ACA or MO (scale bar, 200 μm). Inset, enlarged view of the region in white box (scale bar, 10 μm). Red, tdTomato; blue, DAPI.
Supplementary Figure 7 ACA innervates Pvalb+, Sst+ and Vip+ interneurons and glutamatergic neurons (Camk2α+) in VIS, PTLp and RSP.
(a) Viral vectors and injection procedure for RV-mediated transsynaptic retrograde tracing. (b) Upper panel, viral injection sites in VIS (red box in coronal diagram) of Pvalb-Cre, Sst-Cre and Vip-Cre mice (scale bar, 200 μm). Inset, enlarged view of the region in white box showing starter cells (yellow; scale bar, 20 μm). Lower panel, fluorescence images of ACA and MO (red box in coronal diagram) showing RV-labeled input neurons (green) to each subtype of interneurons in VIS (scale bar, 200 μm). Green, EGFP; red, mCherry; blue, DAPI. (c) Similar to (b), for ACA inputs to PV+, SOM+, VIP+ interneurons and glutamatergic neurons in PTLp. (d) Similar to (b), for ACA inputs to Pvalb+, Sst+, Vip+ interneurons and glutamatergic neurons in RSP. (e) Percentages of input neurons in ACA retrogradely labeled from VIS, PTLp and RSP in Camk2α-Cre (yellow), Pvalb-Cre (blue), Sst-Cre (red) and Vip-Cre mice (green). n = 3 mice in each group. Each circle represents one mouse. Error bar, ± s.e.m.
Supplementary Figure 8 ACA interneurons (Pval+, Sst+ and Vip+) are innervated by VIS, PTLp and RSP.
(a) Left, viral injection sites in ACA (red box in coronal diagram) of Pvalb-Cre, Sst-Cre and Vip-Cre mice (scale bar, 200 μm). Inset, enlarged view of the region in white box showing starter cells (yellow; scale bar, 20 μm). Right, fluorescence images of VIS, PTLp and RSP (red box in coronal diagram) showing RV-labeled input neurons (green) to each subtype of interneurons in ACA (scale bar, 200 μm). Green, EGFP; red, mCherry; blue, DAPI. (b) Percentages of retrogradely labeled input neurons in selected cortical and subcortical brain regions (n = 3 mice in each group). Each circle represents one mouse. Included are the same structures as in Fig. 2h.
Supplementary Figure 9 Whole-brain distributions of axonal projections from MO→SS neurons.
(a) Viral vectors and injection procedure for tracing the axonal projections from MO→SS neurons. (b) Left, bright field image of ACA and MO showing RV-labeled neurons from SS (scale bar, 200 μm). Inset, enlarged view of the region in red box (scale bar, 20 μm). Immunostaining for tdTomato was performed to convert the fluorescence signal (tdTomato expressed by RV) into nickel-enhanced DAB signal; Middle, image of detected axon signals in SS (scale bar, 200 μm); right, bright field image of the region in green box, showing RV-labeled axons of MO→SS neurons (scale bar, 100 μm). (c) Axons detected in all MO→SS samples (scale bar, 1 mm). White masks indicate injection sites excluded from data analysis. (d) Percentages of labeled axons in selected cortical and subcortical brain structures (n = 3 mice). Each circle represents one mouse. Error bar, ±s.e.m. Data for MO axons are the same as in Figure 2D, shown here to facilitate comparison.
Supplementary Figure 10 ACA→VIS and ACA→SC neurons form synapses in the identified cortical and subcortical areas.
(a) Viral vectors and injection procedure for labeling the presynaptic sites of ACA→VIS and ACA→SC neurons. (b) Upper panel, fluorescence images of VIS, PTLp, RSP and STR (red box in coronal diagram) showing axons (green) and putative presynaptic sites (red) from ACA→VIS neurons. Lower panel, similar to upper panel, showing axons (green) and putative presynaptic sites (red) from ACA→SC neurons in RSP, PrL/ILA, LP/LD, STR and SC. Green, mGFP; red, mRuby. Scale bar, 20 μm.
Supplementary Figure 11 WGA-Cre-induced EYFP expression in ACA→VIS and ACA→SC neurons.
(a) Viral vectors and injection procedure for retrograde labeling of ACA→VIS and ACA→SC neurons with EYFP. (b) Left, fluorescence images of AAV-EF1α-mCherry-IRES-WGA-Cre injection site (red) in VIS (upper panel) and SC (lower panel). Right, WGA-Cre-induced EYFP expression (green) in ACA. Note that mCherry fluorescence is located in neuronal somatas at the injection site (left), but not in the ACA (right). White arrowheads, neurons expressing EYFP. Blue, DAPI.
Supplementary Figure 12 WGA-Cre-labeled ACA→VIS and ACA→SC neurons (EYFP+) largely overlapped with Retrobead-labeled ACA→VIS and ACA→SC neurons, respectively.
(a) Viral vectors and retrobead injection procedure for retrograde labeling of ACA→VIS and ACA→SC neurons with EYFP (mediated by WGA-Cre) or red retrobeads. (b) Left, fluorescence images of AAV-EF1α-mCherry-IRES-WGA-Cre and red retrobeads injection site (red) in VIS (upper panel) and SC (lower panel). Middle, retrobeads (red) and WGA-Cre-induced EYFP expression (green) in ACA. Right, enlarged view of the region in white box. White arrowheads, neurons containing both EYFP and retrobeads. Blue, DAPI. Note that most EYFP+ neurons in ACA were also labeled by red retrobeads (VIS injection, 83 ± 4% (s.e.m.), n = 3 mice; SC injection, 80 ± 4%, n = 3 mice).
Supplementary Figure 13 WGA-Cre-labeled ACA→VIS and ACA→SC neurons (EYFP+) showed no overlap with Retrobead-labeled ACA→SC and ACA→VIS neurons, respectively.
(a) Viral vectors and retrobead injection procedure for retrograde labeling of ACA→VIS and ACA→SC neurons with EYFP (mediated by WGA-Cre) or red retrobeads. (b) Upper panel: Left, fluorescence image of AAV-EF1α-mCherry-IRES-WGA-Cre injection site (red) in VIS. Middle, red retrobead injection site in SC. Right, retrobeads (red) and WGA-Cre-induced EYFP expression (green) in ACA. Enlarged view is from the region in white box. Lower panel, similar to upper panel, but the AAV was injected in SC and red retrobeads were injected in VIS. Green arrowheads, neurons expressing EYFP. Red arrowheads, neurons containing red retrobeads. Blue, DAPI. Note that the EYFP+ neurons in ACA were not labeled by red retrobeads (0%, n = 3 mice in each group).
Supplementary Figure 14 Control experiments for WGA-Cre-mediated trans-synaptic tracing in ACA.
(a) Viral vectors and injection procedure for negative control. AAV expressing WGA-Cre was injected into VIS or SC of wild-type mice and AAV with Cre-dependent expression of TVA receptors was injected into the ACA, followed by injection of RV expressing EGFP into ACA. Note that AAV with Cre-dependent expression of rabies glycoprotein was omitted in this control experiment. (b) Left, fluorescence images of AAV-EF1α-mCherry-IRES-WGA-Cre injection site (red) in VIS (upper panel) and SC (lower panel). Middle, injection site of other AAV and RV in ACA containing AAV/RV infected neurons (green). Right, without rabies glycoprotein, no RV-labeled neurons were found in frontal cortical regions such as PL/ILA. Note that due to the leaky TVA expression (Cre-independent)19,21, the RV labeled neurons were not restricted in the layer 5 of ACA in the group with WGA-Cre expression in SC. Since leaky expression of rabies glycoprotein is unlikely sufficient to reconstitute infectious RV particles, this does not compromise long-distance tracing. (c) Schematic of the whole-brain distribution of RV-labeled neurons without rabies glycoprotein. RV-labeled neurons were located in the regions within 700 mm anterior and posterior to the center of injection site in ACA (Bregma +0.3 mm), indicated by green shading.
Supplementary Figure 15 Whole-brain distributions of inputs to MO→SS neurons.
(a) Viral vectors and injection procedure for RV-mediated transsynaptic retrograde tracing from MO→SS neurons. (b) Left, fluorescence images of AAV-EF1α-mCherry-IRES-WGA-Cre injection site in SS (scale bar, 1 mm). Middle, injection site of other AAVs and RV in MO (scale bar, 1 mm). Inset, enlarged view of the region in white box showing AAV/RV infected neurons (green, scale bar, 20 μm). Right, retrogradely labeled neurons (green) in SS (scale bar, 200 μm). Inset, enlarged view of the region in white box (scale bar, 20 μm). Green, EGFP; red, mCherry; blue, DAPI. (c) RV-labeled neurons detected in all MO→SS samples. White masks indicated the injection sites excluded from data analysis (scale bar, 1 mm). (d) Percentages of retrogradely labeled neurons in selected cortical and subcortical brain structures (MO→SS, n = 4 mice). Each circle represents one mouse. Error bar, ±s.e.m. Data for MO inputs are the same as in Figure 3D.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 (PDF 3310 kb)
Supplementary Table 1: Whole brain distribution of inputs and outputs of sensory and frontal cortices
Percentage is calculated as the number of RV-labeled neurons/length of labeled axons in each structure divided by the total number of RV-labeled neurons/total length of labeled axons in the whole brain. Normalized density is calculated as the density in each structure (number of labeled neurons/length of labeled axons divided by volume) normalized by the total number of labeled neurons/total length of labeled axons in the whole brain (XLS 2335 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Xu, M., Chang, WC. et al. Organization of long-range inputs and outputs of frontal cortex for top-down control. Nat Neurosci 19, 1733–1742 (2016). https://doi.org/10.1038/nn.4417
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4417
This article is cited by
-
Adenosine-independent regulation of the sleep–wake cycle by astrocyte activity
Cell Discovery (2023)
-
A frontal transcallosal inhibition loop mediates interhemispheric balance in visuospatial processing
Nature Communications (2023)
-
Continuous multiplexed population representations of task context in the mouse primary visual cortex
Nature Communications (2023)
-
Emergence of cortical network motifs for short-term memory during learning
Nature Communications (2023)
-
The Secondary Motor Cortex-striatum Circuit Contributes to Suppressing Inappropriate Responses in Perceptual Decision Behavior
Neuroscience Bulletin (2023)