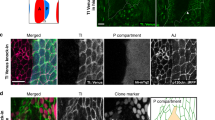
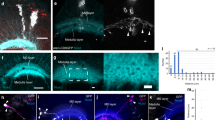
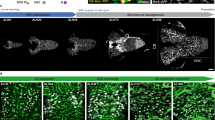
Abstract
Interactions between neurons and glia are a key feature during the assembly of the nervous system. During development, glial cells often follow extending axons, implying that axonal outgrowth and glial migration are precisely coordinated. We found that the anaphase-promoting complex/cyclosome (APC/C) co-activator fizzy-related/Cdh1 (Fzr/Cdh1) is involved in the non-autonomous control of peripheral glial migration in postmitotic Drosophila neurons. APC/CFzr/Cdh1 is a cell-cycle regulator that targets proteins that are required for G1 arrest for ubiquitination and subsequent degradation. We found that Fzr/Cdh1 function is mediated by the immunoglobulin superfamily cell adhesion molecule Fasciclin2 (Fas2). In motor neurons Fzr/Cdh1 is crucial for the establishment of a graded axonal distribution of Fas2. Axonal Fas2 interacts homophilically with a glial isoform of Fas2. Glial migration is initiated along axonal segments that have low levels of Fas2 but stalls in axonal domains with high levels of Fas2 on their surfaces. This represents a simple mechanism by which a subcellular gradient of adhesiveness can coordinate glial migration with axonal growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nave, K.A. & Trapp, B.D. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 31, 535–561 (2008).
Freeman, M.R. & Doherty, J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 29, 82–90 (2006).
Halassa, M.M. & Haydon, P.G. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 72, 335–355 (2010).
Klämbt, C. Modes and regulation of glial migration in vertebrates and invertebrates. Nat. Rev. Neurosci. 10, 769–779 (2009).
Lyons, D.A. et al. erbb3 and erbb2 are essential for Schwann cell migration and myelination in zebrafish. Curr. Biol. 15, 513–524 (2005).
Gilmour, D.T., Maischein, H.M. & Nusslein-Volhard, C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron 34, 577–588 (2002).
Kucenas, S. et al. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143–151 (2008).
Sepp, K.J., Schulte, J. & Auld, V.J. Developmental dynamics of peripheral glia in Drosophila melanogaster. Glia 30, 122–133 (2000).
Silies, M. et al. Glial cell migration in the eye disc. J. Neurosci. 27, 13130–13139 (2007).
von Hilchen, C.M., Beckervordersandforth, R.M., Rickert, C., Technau, G.M. & Altenhein, B. Identity, origin, and migration of peripheral glial cells in the Drosophila embryo. Mech. Dev. 125, 337–352 (2008).
Edenfeld, G. et al. Notch and Numb are required for normal migration of peripheral glia in Drosophila. Dev. Biol. 301, 27–37 (2007).
Sepp, K.J. & Auld, V.J. RhoA and Rac1 GTPases mediate the dynamic rearrangement of actin in peripheral glia. Development 130, 1825–1835 (2003).
Edenfeld, G. et al. The splicing factor crooked neck associates with the RNA-binding protein HOW to control glial cell maturation in Drosophila. Neuron 52, 969–980 (2006).
Freeman, M.R., Delrow, J., Kim, J., Johnson, E. & Doe, C.Q. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 38, 567–580 (2003).
von Hilchen, C.M., Hein, I., Technau, G.M. & Altenhein, B. Netrins guide migration of distinct glial cells in the Drosophila embryo. Development 137, 1251–1262 (2010).
Sigrist, S.J. & Lehner, C.F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671–681 (1997).
Hummel, T., Schimmelpfeng, K. & Klämbt, C. Commissure formation in the embryonic CNS of Drosophila. Dev. Biol. 209, 381–398 (1999).
Reber, A., Lehner, C.F. & Jacobs, H.W. Terminal mitoses require negative regulation of Fzr/Cdh1 by Cyclin A, preventing premature degradation of mitotic cyclins and String/Cdc25. Development 133, 3201–3211 (2006).
Berger, C., Renner, S., Luer, K. & Technau, G.M. The commonly used marker ELAV is transiently expressed in neuroblasts and glial cells in the Drosophila embryonic CNS. Dev. Dyn. 236, 3562–3568 (2007).
Peters, J.M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 (2006).
Bischof, J., Maeda, R.K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312–3317 (2007).
Gieffers, C., Peters, B.H., Kramer, E.R., Dotti, C.G. & Peters, J.M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl. Acad. Sci. USA 96, 11317–11322 (1999).
Grenningloh, G., Rehm, E.J. & Goodman, C.S. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67, 45–57 (1991).
Maness, P.F. & Schachner, M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 (2007).
Wright, J.W. & Copenhaver, P.F. Cell type–specific expression of fasciclin II isoforms reveals neuronal-glial interactions during peripheral nerve growth. Dev. Biol. 234, 24–41 (2001).
Higgins, M.R. et al. Different isoforms of fasciclin II are expressed by a subset of developing olfactory receptor neurons and by olfactory-nerve glial cells during formation of glomeruli in the moth Manduca sexta. Dev. Biol. 244, 134–154 (2002).
Buszczak, M. et al. The carnegie protein trap library: a versatile tool for Drosophila developmental studies. Genetics 175, 1505–1531 (2007).
Prag, S. et al. NCAM regulates cell motility. J. Cell Sci. 115, 283–292 (2002).
Cavallaro, U., Niedermeyer, J., Fuxa, M. & Christofori, G. N-CAM modulates tumor-cell adhesion to matrix by inducing FGF-receptor signaling. Nat. Cell Biol. 3, 650–657 (2001).
Paratcha, G., Ledda, F. & Ibanez, C.F. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113, 867–879 (2003).
Diestel, S., Schaefer, D., Cremer, H. & Schmitz, B. NCAM is ubiquitylated, endocytosed and recycled in neurons. J. Cell Sci. 120, 4035–4049 (2007).
Mathew, D., Popescu, A. & Budnik, V. Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J. Neurosci. 23, 10710–10716 (2003).
Juo, P. & Kaplan, J.M. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr. Biol. 14, 2057–2062 (2004).
van Roessel, P., Elliott, D.A., Robinson, I.M., Prokop, A. & Brand, A.H. Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 119, 707–718 (2004).
Konishi, Y., Stegmuller, J., Matsuda, T., Bonni, S. & Bonni, A. Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 (2004).
Stegmüller, J. et al. Cell-intrinsic regulation of axonal morphogenesis by the Cdh1-APC target SnoN. Neuron 50, 389–400 (2006).
Yang, Y. et al. A Cdc20-APC ubiquitin signaling pathway regulates presynaptic differentiation. Science 326, 575–578 (2009).
Dodd, J., Morton, S.B., Karagogeos, D., Yamamoto, M. & Jessell, T.M. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron 1, 105–116 (1988).
Bastiani, M.J., Harrelson, A.L., Snow, P.M. & Goodman, C.S. Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell 48, 745–755 (1987).
Ango, F. et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119, 257–272 (2004).
Katsuki, T., Ailani, D., Hiramoto, M. & Hiromi, Y. Intra-axonal patterning: intrinsic compartmentalization of the axonal membrane in Drosophila neurons. Neuron 64, 188–199 (2009).
Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 9, 26–35 (2008).
Weinhold, B. et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 280, 42971–42977 (2005).
Cremer, H. et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367, 455–459 (1994).
Kirby, B.B. et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9, 1506–1511 (2006).
Wright, J.W. & Copenhaver, P.F. Different isoforms of fasciclin II play distinct roles in the guidance of neuronal migration during insect embryogenesis. Dev. Biol. 225, 59–78 (2000).
Mao, Y. & Freeman, M. Fasciclin 2, the Drosophila orthologue of neural cell-adhesion molecule, inhibits EGF receptor signalling. Development 136, 473–481 (2009).
Lehembre, F. et al. NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 27, 2603–2615 (2008).
Franzdóttir, S.R. et al. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature 460, 758–761 (2009).
Kohsaka, H., Takasu, E. & Nose, A. In vivo induction of postsynaptic molecular assembly by the cell adhesion molecule Fasciclin2. J. Cell Biol. 179, 1289–1300 (2007).
Acknowledgements
We thank C. Lehner, J. Raff, B. Altenhein, T. Orr-Weaver, G. Tear, K. Basler, M. Landgraf and A. Nose for flies and antibodies; T. Hummel, E. Raz, A. Püschel and T.R. Clandinin for comments on the manuscript; G. Edenfeld, K. Krukkert, N. Otto, A. Weinbrecht and H. Neuert for help with initial experiments; J. Lipp for help with statistical analysis; and members of the Klämbt lab for support and discussions. This work was supported by a PhD fellowship of the Boehringer Ingelheim Fonds to M.S. and grants of the Deutsche Forschungsgemeinschaft to C.K.
Author information
Authors and Affiliations
Contributions
M.S. conducted the experiments and analysed the data. C.K. contributed ideas. M.S. and C.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 6519 kb)
Supplementary Movie 1
Wild type embryo carrying two repoGal4 and two UAS-GFPstinger transgenes. (MOV 1353 kb)
Supplementary Movie 2
fzr/cdh1ie28 mutant type embryo carrying two repoGal4 and two UAS-GFPstinger transgenes. (MOV 3612 kb)
Rights and permissions
About this article
Cite this article
Silies, M., Klämbt, C. APC/CFzr/Cdh1-dependent regulation of cell adhesion controls glial migration in the Drosophila PNS. Nat Neurosci 13, 1357–1364 (2010). https://doi.org/10.1038/nn.2656
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2656
This article is cited by
-
JNK signaling in pioneer neurons organizes ventral nerve cord architecture in Drosophila embryos
Nature Communications (2023)
-
Drosophila ßHeavy-Spectrin is required in polarized ensheathing glia that form a diffusion-barrier around the neuropil
Nature Communications (2021)
-
Mechanisms for the temporal regulation of substrate ubiquitination by the anaphase-promoting complex/cyclosome
Cell Division (2019)
-
Ubiquitylation at the crossroads of development and disease
Nature Reviews Molecular Cell Biology (2018)
-
The cell adhesion molecule Fasciclin2 regulates brush border length and organization in Drosophila renal tubules
Nature Communications (2016)