Abstract
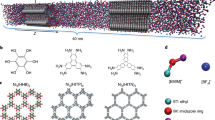
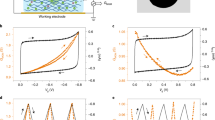
Supercapacitors store charge through the electrosorption of ions on microporous electrodes. Despite major efforts to understand this phenomenon, a molecular-level picture of the electrical double layer in working devices is still lacking as few techniques can selectively observe the ionic species at the electrode/electrolyte interface. Here, we use in situ NMR to directly quantify the populations of anionic and cationic species within a working microporous carbon supercapacitor electrode. Our results show that charge storage mechanisms are different for positively and negatively polarized electrodes for the electrolyte tetraethylphosphonium tetrafluoroborate in acetonitrile; for positive polarization charging proceeds by exchange of the cations for anions, whereas for negative polarization, cation adsorption dominates. In situ electrochemical quartz crystal microbalance measurements support the NMR results and indicate that adsorbed ions are only partially solvated. These results provide new molecular-level insight, with the methodology offering exciting possibilities for the study of pore/ion size, desolvation and other effects on charge storage in supercapacitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Salitra, G., Soffer, A., Eliad, L., Cohen, Y. & Aurbach, D. Carbon electrodes for double layer capacitors. I. Relations between ion and pore dimensions. J. Electrochem. Soc. 147, 2486–2493 (2000).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006).
Largeot, C. et al. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
Vix-Guterl, C. et al. Electrochemical energy storage in ordered porous carbon materials. Carbon 43, 1293–1302 (2005).
Chmiola, J., Largeot, C., Taberna, P.-L., Simon, P. & Gogotsi, Y. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew. Chem. Int. Ed. 47, 3392–3395 (2008).
Fedorov, M. V. & Kornyshev, A. A. Ionic liquids at electrified interfaces. Chem. Rev. 114, 2978–3036 (2014).
Burt, R., Birkett, G. & Zhao, X. S. A review of molecular modeling of electric double layer capacitors. Phys. Chem. Chem. Phys. 16, 6519–6538 (2014).
Feng, G. & Cummings, P. T. Supercapacitor capacitance exhibits oscillatory behaviour as a function of nanopore size. J. Phys. Chem. Lett. 2, 2859–2864 (2011).
Wu, P., Huang, J., Meunier, V., Sumpter, B. G. & Qiao, R. Complex capacitance scaling in ionic liquids-filled nanopores. ACS Nano 5, 9044–9051 (2011).
Kondrat, S. & Kornyshev, A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 23, 022201 (2011).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nature Mater. 11, 306–310 (2012).
Merlet, C. et al. Highly confined ions store charge more efficiently in supercapacitors. Nature Commun. 4, 2701 (2013).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nature Mater. 13, 387–393 (2014).
Levi, M. D., Salitra, G., Levy, N., Aurbach, D. & Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nature Mater. 8, 872–875 (2009).
Tsai, W.-Y., Taberna, P.-L. & Simon, P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons. J. Am. Chem. Soc. 136, 8722–8728 (2014).
Richey, F. W., Dyatkin, B., Gogotsi, Y. & Elabd, Y. A. Ion dynamics in porous carbon electrodes in supercapacitors using in situ infrared spectroelectrochemistry. J. Am. Chem. Soc. 135, 12818–12826 (2013).
Richey, F. W., Tran, C., Kalra, V. & Elabd, Y. A. Ionic liquid dynamics in nanoporous carbon nanofibers in supercapacitors measured with in operando infrared spectroelectrochemistry. J. Phys. Chem. C 118, 21846–21855 (2014).
Forse, A. C. et al. Nuclear magnetic resonance study of ion adsorption on microporous carbide-derived carbon. Phys. Chem. Chem. Phys. 15, 7722–7730 (2013).
Borchardt, L., Oschatz, M., Paasch, S., Kaskel, S. & Brunner, E. Interaction of electrolyte molecules with carbon materials of well-defined porosity: Characterization by solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 15, 15177–15184 (2013).
Deschamps, M. et al. Exploring electrolyte organization in supercapacitor electrodes with solid-state NMR. Nature Mater. 12, 351–358 (2013).
Wang, H. et al. Real-time NMR studies of electrochemical double-layer capacitors. J. Am. Chem. Soc. 133, 19720–19273 (2011).
Wang, H. et al. In situ NMR spectroscopy of supercapacitors: Insight into the charge storage mechanism. J. Am. Chem. Soc. 135, 18968–18980 (2013).
Griffin, J. M. et al. Ion counting in supercapacitor electrodes using NMR spectroscopy. Faraday Discuss. 176, 49–68 (2014).
Ilott, A. J., Trease, N. M., Grey, C. P. & Jerschow, A. Multinuclear in situ magnetic resonance imaging of electrochemical double-layer capacitors. Nature Commun. 5, 4536 (2014).
Forse, A. C., Griffin, J. M., Presser, V., Gogotsi, Y. & Grey, C. P. Ring current effects: Factors affecting the NMR chemical shift of molecules adsorbed on porous carbons. J. Phys. Chem. C 118, 7508–7514 (2014).
Kim, Y.-J., Masutzawa, Y., Ozaki, S., Endo, M. & Dresselhaus, M. S. PVDC-based carbon material by chemical activation and its application to nonaqueous EDLC. J. Electrochem. Soc. 151, E199–E205 (2004).
Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung [The use of resonance quartz crystals for weighing thin layers and as a microbalance]. Z. Phys. 155, 206–222 (1959).
Levi, M. D. et al. Electrochemical quartz crystal microbalance (EQCM) studies of ions and solvents insertion into highly porous activated carbons. J. Am. Chem. Soc. 132, 13220–13222 (2010).
Levi, M. D., Sigalov, S., Aurbach, D. & Daikhin, L. In situ electrochemical quartz crystal admittance methodology for tracking compositional and mechanical changes in porous carbon electrodes. J. Phys. Chem. C 117, 14876–14889 (2013).
Fukano, M. et al. Vertically oriented propylene carbonate molecules and tetraethyl ammonium ions in carbon slit pores. J. Phys. Chem. C 117, 5752–5757 (2013).
Hantel, M. M., Presser, V., Kötz, R. & Gogotsi, Y. In situ electrochemical dilatometry of carbide-derived carbons. Electrochem. Commun. 13, 1221–1224 (2011).
Kondrat, S., Georgi, N., Fedorov, M. V. & Kornyshev, A. A. A superionic state in nano-porous double-layer capacitors: Insights from Monte Carlo simulations. Phys. Chem. Chem. Phys. 13, 11359–11366 (2011).
Acknowledgements
A.C.F., J.M.G. and C.P.G. acknowledge the Sims Scholarship (A.C.F.), EPSRC (through the Supergen consortium for J.M.G.) and the EU ERC (through an Advanced Fellowship to C.P.G.) for financial support. P.S. and W.-Y.T. acknowledge support from the European Research Council (ERC, Advanced Grant, ERC-2011-AdG, Project 291543–IONACES). P.S. also acknowledges financial support from the Chair ‘Embedded Multi-Functional Nanomaterials’ from the Airbus Group Foundation. A.C.F. and J.M.G. thank the NanoDTC Cambridge for travel funding. We also thank A. Kornyshev, S. Kondrat and C. Merlet for useful discussions.
Author information
Authors and Affiliations
Contributions
J.M.G., A.C.F. and C.P.G. designed the research. J.M.G. made supercapacitor cells and electrolytes, performed NMR experiments and analysed the NMR data. W.-Y.T., P.-L.T. and P.S. designed the EQCM work. W.-Y.T. carried out the EQCM experiments and W.-Y.T., P.-L.T. and P.S. analysed the data. All authors contributed to discussion of the data and writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1837 kb)
Rights and permissions
About this article
Cite this article
Griffin, J., Forse, A., Tsai, WY. et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nature Mater 14, 812–819 (2015). https://doi.org/10.1038/nmat4318
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4318
This article is cited by
-
Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors
Nature Communications (2023)
-
Functionalizing the interfacial double layer to enable uniform zinc deposition
Science China Chemistry (2023)
-
Developing in situ electron paramagnetic resonance characterization for understanding electron transfer of rechargeable batteries
Nano Research (2023)
-
Critical role of water structure around interlayer ions for ion storage in layered double hydroxides
Nature Communications (2022)
-
Microstructural evolution of N–O–S self-doped porous carbons based on heteroatomic migration for supercapacitor electrodes
Journal of Materials Science: Materials in Electronics (2022)