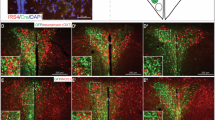
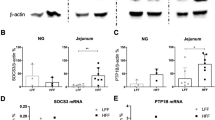
Abstract
Inflammation has a critical role in the development of insulin resistance. Recent evidence points to a contribution by the central nervous system in the modulation of peripheral inflammation through the anti-inflammatory reflex. However, the importance of this phenomenon remains elusive in type 2 diabetes pathogenesis. Here we show that rat insulin-2 promoter (Rip)-mediated deletion of Pten, a gene encoding a negative regulator of PI3K signaling, led to activation of the cholinergic anti-inflammatory pathway that is mediated by M2 activated macrophages in peripheral tissues. As such, Rip-cre+ Ptenflox/flox mice showed lower systemic inflammation and greater insulin sensitivity under basal conditions compared to littermate controls, which were abolished when the mice were treated with an acetylcholine receptor antagonist or when macrophages were depleted. After feeding with a high-fat diet, the Pten-deleted mice remained markedly insulin sensitive, which correlated with massive subcutaneous fat expansion. They also exhibited more adipogenesis with M2 macrophage infiltration, both of which were abolished after disruption of the anti-inflammatory efferent pathway by left vagotomy. In summary, we show that Pten expression in Rip+ neurons has a critical role in diabetes pathogenesis through mediating the anti-inflammatory reflex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Shoelson, S.E., Lee, J. & Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Osborn, O. & Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 (2012).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Kanda, H. et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 (2006).
Weisberg, S.P. et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 (2006).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Van Ginderachter, J.A. et al. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211, 487–501 (2006).
Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 (2009).
Andersson, U. & Tracey, K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 30, 313–335 (2012).
Sternberg, E.M. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6, 318–328 (2006).
Maier, S.F., Goehler, L.E., Fleshner, M. & Watkins, L.R. The role of the vagus nerve in cytokine-to-brain communication. Ann. NY Acad. Sci. 840, 289–300 (1998).
Groves, D.A. & Brown, V.J. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 29, 493–500 (2005).
Borovikova, L.V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Bernik, T.R. et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 (2002).
Saeed, R.W. et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J. Exp. Med. 201, 1113–1123 (2005).
Huston, J.M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 (2006).
de Jonge, W.J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851 (2005).
Kreier, F. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat—functional implications. J. Clin. Invest. 110, 1243–1250 (2002).
Watkins, L.R. & Maier, S.F. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J. Intern. Med. 257, 139–155 (2005).
The, F. et al. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental post-operative ileus. Br. J. Pharmacol. 163, 1007–1016 (2011).
Choudhury, A.I. et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. J. Clin. Invest. 115, 940–950 (2005).
Wang, L. et al. Deletion of Pten in pancreatic β-cells protects against deficient β-cell mass and function in mouse models of type 2 diabetes. Diabetes 59, 3117–3126 (2010).
Song, J., Xu, Y., Hu, X., Choi, B. & Tong, Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis 48, 628–634 (2010).
Wicksteed, B. et al. Conditional gene targeting in mouse pancreatic β-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 59, 3090–3098 (2010).
Backman, S.A. et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29, 396–403 (2001).
Nguyen, K.T. et al. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol. Cell. Biol. 26, 4511–4518 (2006).
Petersen, A.M. & Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 98, 1154–1162 (2005).
Petersen, A.M. & Pedersen, B.K. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J. Physiol. Pharmacol. 57 (suppl. 10), 43–51 (2006).
Kubota, N. et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J. Clin. Invest. 114, 917–927 (2004).
Stanger, B.Z. et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8, 185–195 (2005).
Cantley, J. et al. Pancreatic deletion of insulin receptor substrate 2 reduces beta and alpha cell mass and impairs glucose homeostasis in mice. Diabetologia 50, 1248–1256 (2007).
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Bourlier, V. et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117, 806–815 (2008).
Plum, L. et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J. Clin. Invest. 116, 1886–1901 (2006).
Münzberg, H. & Myers, M.G. Jr. Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 8, 566–570 (2005).
Rulifson, E.J., Kim, S.K. & Nusse, R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118–1120 (2002).
Kodama, E. et al. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 20, 2955–2960 (2006).
Gannon, M., Shiota, C., Postic, C., Wright, C.V. & Magnuson, M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26, 139–142 (2000).
Plum, L. et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 6, 431–445 (2007).
Odegaard, J.I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Odegaard, J.I. et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 (2008).
Bouhlel, M.A. et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6, 137–143 (2007).
Winer, S. et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929 (2009).
Winer, D.A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Mauer, J. et al. Myeloid cell–restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 6, e1000938 (2010).
Andersson, K. & Arner, P. Cholinoceptor-mediated effects on glycerol output from human adipose tissue using in situ microdialysis. Br. J. Pharmacol. 115, 1155–1162 (1995).
Olofsson, P.S. et al. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow–derived non-T cells is required for the inflammatory reflex. Mol. Med. 18, 539–543 (2012).
Kim, J.Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Primeau, V. et al. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. (Lond.) 35, 971–981 (2011).
Karelis, A.D. et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 90, 4145–4150 (2005).
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell–specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Wijesekara, N. et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol. Cell. Biol. 25, 1135–1145 (2005).
Nguyen, K.T. et al. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol. Cell. Biol. 26, 4511–4518 (2006).
Choi, D. et al. Erythropoietin protects against diabetes through direct effects on pancreatic beta cells. J. Exp. Med. 207, 2831–2842 (2010).
Münzberg, H. et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J. Neurosci. 27, 69–74 (2007).
Franklin, K.B.J. & Paxinos, G. The Mouse Brain in Sterotaxic Coordinates (Academic Press, New York, 2008).
Acknowledgements
We thank Y. Dor (Department of Developmental Biology and Cancer Research, The Institute for Medical Research Israel-Canada, The Hebrew University-Hadassah Medical School) for providing the Pdx1-Cre-ER mice. This work was supported by grants to M.W. from the Canadian Institutes of Health Research (CIHR; MOP-93707) and the Canadian Diabetes Association (CDA). M.W. holds a Canada Research Chair in Signal Transduction in Diabetes Pathogenesis. M.G.M.Jr. was supported by a grant from the US National Institutes of Health (DK57768). Support was also received from grants to D.A.W. from the CIHR (MOP-119414) and CDA. L.W. was supported by Frederick Banting and Charles Best Canada Graduate Scholarship from CIHR, a Novo Nordisk Graduate Scholarship from the Banting and Best Diabetes Centre, a Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) and a Comprehensive Research Experience for Medical Students (CREMS) Scholarship from the Faculty of Medicine, University of Toronto. S.T. was supported by a Toronto General Research Institute Postdoctoral Fellowship. C.T.L. is supported by the Eliot Phillipson Clinician Scientist Training Program and a Banting and Best Diabetes Centre Postdoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
L.W. performed experiments, analyzed data, wrote the manuscript and contributed to the study concept. D.O., S.T., M.B.A., A.J.E. and C.F. performed experiments, analyzed data and prepared data for presentation. C.T.L. analyzed data and edited the manuscript. S.A.S. developed methods and animal models and performed experiments. A.S. and T.W.M. developed animal models. C.J.P., D.A.W. and M.G.M.Jr. contributed to study design, discussion and the manuscript. M.W. supervised the project, developed the study concept and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 4660 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Opland, D., Tsai, S. et al. Pten deletion in RIP-Cre neurons protects against type 2 diabetes by activating the anti-inflammatory reflex. Nat Med 20, 484–492 (2014). https://doi.org/10.1038/nm.3527
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3527
This article is cited by
-
Brief periods of transcutaneous auricular vagus nerve stimulation improve autonomic balance and alter circulating monocytes and endothelial cells in patients with metabolic syndrome: a pilot study
Bioelectronic Medicine (2023)
-
Macrophage Jak2 deficiency accelerates atherosclerosis through defects in cholesterol efflux
Communications Biology (2022)
-
The oxylipin profile is associated with development of type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY)
Diabetologia (2021)
-
STAT3 dictates β-cell apoptosis by modulating PTEN in streptozocin-induced hyperglycemia
Cell Death & Differentiation (2020)
-
FAK signalling controls insulin sensitivity through regulation of adipocyte survival
Nature Communications (2017)