Abstract
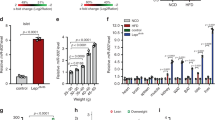
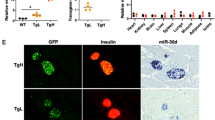
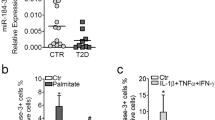
Beta-cell dysfunction and impaired insulin production are hallmarks of diabetes1, but despite the growing diabetes epidemic, the molecular mechanisms underlying this disease have remained unclear. We identified thioredoxin-interacting protein (TXNIP), a cellular redox regulator, as a crucial factor in beta-cell biology and show that beta-cell TXNIP is upregulated in diabetes, whereas TXNIP deficiency protects against diabetes by preventing beta-cell apoptosis2,3. Here we show that TXNIP and diabetes induce beta-cell expression of a specific microRNA, miR-204, which in turn blocks insulin production by directly targeting and downregulating MAFA, a known insulin transcription factor. In particular, we first discovered the regulation of miR-204 by TXNIP by microarray analysis, followed by validation studies in INS-1 beta cells, islets of Txnip-deficient mice, diabetic mouse models and primary human islets. We then further found that TXNIP induces miR-204 by inhibiting the activity of signal transducer and activator of transcription 3 (STAT3), a transcription factor that is involved in miR-204 regulation4,5. We also identified MAFA as a target that is downregulated by miR-204. Taken together, our results demonstrate that TXNIP controls microRNA expression and insulin production and that miR-204 is involved in beta-cell function. The newly identified TXNIP–miR-204–MAFA–insulin pathway may contribute to diabetes progression and provides new insight into TXNIP function and microRNA biology in health and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Poitout, V. & Robertson, R.P. Minireview: secondary beta-cell failure in type 2 diabetes—a convergence of glucotoxicity and lipotoxicity. Endocrinology 143, 339–342 (2002).
Chen, J., Saxena, G., Mungrue, I.N., Lusis, A.J. & Shalev, A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta cell apoptosis. Diabetes 57, 938–944 (2008).
Chen, J. et al. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta cell mass and protects against diabetes. FASEB J. 22, 3581–3594 (2008).
Courboulin, A. et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 208, 535–548 (2011).
Paulin, R. et al. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 301, H1798–H1809 (2011).
Nishiyama, A., Masutani, H., Nakamura, H., Nishinaka, Y. & Yodoi, J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life 52, 29–33 (2001).
Shalev, A. et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFβ signaling pathway. Endocrinology 143, 3695–3698 (2002).
Minn, A.H., Hafele, C. & Shalev, A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 146, 2397–2405 (2005).
Minn, A.H. et al. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem. Biophys. Res. Commun. 336, 770–778 (2005).
Saxena, G., Chen, J. & Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 285, 3997–4005 (2010).
Chen, J., Fontes, G., Saxena, G., Poitout, V. & Shalev, A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid–induced ER stress-mediated beta-cell death. Diabetes 59, 440–447 (2010).
Sun, Y. et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 32, e188 (2004).
Landgraf, P. et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129, 1401–1414 (2007).
Winter, J., Jung, S., Keller, S., Gregory, R.I. & Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11, 228–234 (2009).
Fernandez-Valverde, S.L., Taft, R.J. & Mattick, J.S. MicroRNAs in beta-cell biology, insulin resistance, diabetes and its complications. Diabetes 60, 1825–1831 (2011).
Kantharidis, P., Wang, B., Carew, R.M. & Lan, H.Y. Diabetes complications: the microRNA perspective. Diabetes 60, 1832–1837 (2011).
Lynn, F.C. et al. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56, 2938–2945 (2007).
Melkman-Zehavi, T. et al. miRNAs control insulin content in pancreatic beta-cells via downregulation of transcriptional repressors. EMBO J. 30, 835–845 (2011).
Poy, M.N. et al. A pancreatic islet–specific microRNA regulates insulin secretion. Nature 432, 226–230 (2004).
Poy, M.N. et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc. Natl. Acad. Sci. USA 106, 5813–5818 (2009).
Tattikota, S.G. & Poy, M.N. Re-dicing the pancreatic beta-cell: do microRNAs define cellular identity? EMBO J. 30, 797–799 (2011).
Roldo, C. et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 24, 4677–4684 (2006).
Krol, J. et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631 (2010).
Xu, G., Chen, J., Jing, G. & Shalev, A. Preventing beta-cell loss and diabetes with calcium channel blockers. Diabetes 61, 848–856 (2012).
Clee, S.M., Nadler, S.T. & Attie, A.D. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am. J. Ther. 12, 491–498 (2005).
Moitra, J. et al. Life without white fat: a transgenic mouse. Genes Dev. 12, 3168–3181 (1998).
Artner, I. et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 59, 2530–2539 (2010).
Le Lay, J. & Stein, R. Involvement of PDX-1 in activation of human insulin gene transcription. J. Endocrinol. 188, 287–294 (2006).
Sharma, A. et al. The NeuroD1/BETA2 sequences essential for insulin gene transcription colocalize with those necessary for neurogenesis and p300/CREB binding protein binding. Mol. Cell Biol. 19, 704–713 (1999).
Vanhoose, A.M. et al. MafA and MafB regulate Pdx1 transcription through the area II control region in pancreatic beta cells. J. Biol. Chem. 283, 22612–22619 (2008).
Matsuoka, T.A. et al. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes 59, 1709–1720 (2010).
Vanderford, N.L., Andrali, S.S. & Ozcan, S. Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway. J. Biol. Chem. 282, 1577–1584 (2007).
Lewis, B.P., Burge, C.B. & Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 (2005).
Rani, S. et al. Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol. Biochem. 25, 667–674 (2010).
Zhang, C. et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell Biol. 25, 4969–4976 (2005).
Kostromina, E. et al. Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas. Endocrinology 151, 2050–2059 (2010).
Bolmeson, C. et al. Differences in islet-enriched miRNAs in healthy and glucose intolerant human subjects. Biochem. Biophys. Res. Commun. 404, 16–22 (2011).
El Ouaamari, A. et al. miR-375 targets 3′-phosphoinositide–dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes 57, 2708–2717 (2008).
Zhao, X., Mohan, R., Ozcan, S. & Tang, X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic beta-cells. J. Biol. Chem. 287, 31155–31164 (2012).
Krützfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Cha-Molstad, H., Saxena, G., Chen, J. & Shalev, A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element–binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J. Biol. Chem. 284, 16898–16905 (2009).
Acknowledgements
This work was supported by grants to A.S. from the US National Institutes of Health (R01DK-078752), the American Diabetes Association (7-12-BS-167) and the Juvenile Diabetes Research Foundation and JNJSI (40-2011-1). A-ZIP/F mice were a generous gift of C. Vinson, US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
G.X. designed, performed and analyzed the experiments and helped prepare the manuscript. J.C. was responsible for the mouse studies and islet isolations. G.J. performed most of the cloning and helped with some of the experiments. A.S. conceived the project, supervised the work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–2 (PDF 668 kb)
Rights and permissions
About this article
Cite this article
Xu, G., Chen, J., Jing, G. et al. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med 19, 1141–1146 (2013). https://doi.org/10.1038/nm.3287
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3287
This article is cited by
-
Verapamil-loaded supramolecular hydrogel patch attenuates metabolic dysfunction-associated fatty liver disease via restoration of autophagic clearance of aggregated proteins and inhibition of NLRP3
Biomaterials Research (2023)
-
The role of TXNIP in cancer: a fine balance between redox, metabolic, and immunological tumor control
British Journal of Cancer (2023)
-
Childhood obesity, metabolic syndrome, and oxidative stress: microRNAs go on stage
Reviews in Endocrine and Metabolic Disorders (2023)
-
iPSC-Derived Pancreatic Progenitors Lacking FOXA2 Reveal Alterations in miRNA Expression Targeting Key Pancreatic Genes
Stem Cell Reviews and Reports (2023)
-
Epigenetics: key to improve delayed wound healing in type 2 diabetes
Molecular and Cellular Biochemistry (2022)