Abstract
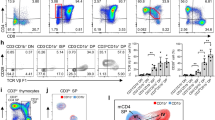
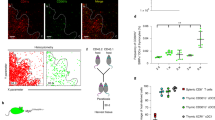
CD1d-reactive NKT cells are a separate T cell sublineage. Instructive models propose that NKT cells branch off the mainstream developmental pathway because of their T cell antigen receptor specificity, whereas stochastic models would propose that they develop from precursor cells committed to this sublineage before variable-gene rearrangement. We show here that immature double-positive (DP) thymocytes form the canonical rearranged Vα gene of NKT cells at nearly equivalent frequencies in the presence or absence of CD1d expression. After interacting with CD1d in the thymus, these cells give rise to expanded populations of NKT cells—including both CD4+ and double-negative lymphocytes in the thymus and periphery—that express this α chain. These results confirm the existence of a DP intermediate for CD1d-reactive NKT cells. They also show that the early developmental stages of these T cells are not governed by a distinct mechanism, which is consistent with the TCR-instructive model of differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
MacDonald, H. R. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J. Exp. Med. 182, 633–638 (1995).
Bendelac, A., Rivera, M. N., Park, S. H. & Roark, J. H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15, 535–562 (1997).
Godfrey, D. I., Hammond, K. J., Poulton, L. D., Smyth, M. J. & Baxter, A. G. NKT cells: facts, functions and fallacies. Immunol. Today 21, 573–583 (2000).
Eberl, G. et al. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162, 6410–6419 (1999).
Hammond, K. J. et al. NKT cells are phenotypically and functionally diverse. Eur. J. Immunol. 29, 3768–3781 (1999).
Arase, H., Arase, N., Ogasawara, K., Good, R. A. & Onoe, K. An NK1.1+ CD4+8− single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor Vβ family. Proc. Natl Acad. Sci. USA 89, 6506–6510 (1992).
Lantz, O. & Bendelac, A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180, 1097–1106 (1994).
Makino, Y., Kanno, R., Ito, T., Higashino, K. & Taniguchi, M. Predominant expression of invariant Vα14+ TCRα chain in NK1.1+ T cell populations. Int. Immunol. 7, 1157–1161 (1995).
Ohteki, T. & MacDonald, H. R. Stringent Vβ requirement for the development of NK1.1+ T cell receptor-α/β+ cells in mouse liver. J. Exp. Med. 183, 1277–1282 (1996).
Shimamura, M., Ohteki, T., Beutner, U. & MacDonald, H. R. Lack of directed Vα14-Jα281 rearrangements in NK1+ T cells. Eur. J. Immunol. 27, 1576–1579 (1997).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Burdin, N. et al. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J. Immunol. 161, 3271–3281 (1998).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Matsuda, J. L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
Cardell, S. et al. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182, 993–1004 (1995).
Behar, S. M., Podrebarac, T. A., Roy, C. J., Wang, C. R. & Brenner, M. B. Diverse TCRs recognize murine CD1. J. Immunol. 162, 161–167 (1999).
Park, S.-H. et al. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193, 893–904 (2001).
Bix, M., Coles, M. & Raulet, D. Positive selection of Vβ8+ CD4−8− thymocytes by class I molecules expressed by hematopoietic cells. J. Exp. Med. 178, 901–908 (1993).
Coles, M. C. & Raulet, D. H. Class I dependence of the development of CD4+ CD8− NK1.1+ thymocytes. J. Exp. Med. 180, 395–399 (1994).
Bendelac, A., Killeen, N., Littman, D. R. & Schwartz, R. H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263, 1774–1778 (1994).
Ohteki, T. & MacDonald, H. R. Major histocompatibility complex class I related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 180, 699–704 (1994).
Coles, M. C. & Raulet, D. H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Alberola-Ila, J., Hogquist, K. A., Swan, K. A., Bevan, M. J. & Perlmutter, R. M. Positive and negative selection invoke distinct signaling pathways. J. Exp. Med. 184, 9–18 (1996).
Eberl, G., Lowin-Kropf, B. & MacDonald, H. R. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J. Immunol. 163, 4091–4094 (1999).
Gadue, P., Morton, N. & Stein, P. L. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J. Exp. Med. 190, 1189–1196 (1999).
Walunas, T. L., Wang, B., Wang, C. R. & Leiden, J. M. Cutting edge: the Ets1 transcription factor is required for the development of NK T cells in mice. J. Immunol. 164, 2857–2860 (2000).
Ohteki, T., Ho, S., Suzuki, H., Mak, T. W. & Ohashi, P. S. Role for IL-15/IL-15 receptor β-chain in natural killer 1.1+ T cell receptor-αβ+ cell development. J. Immunol. 159, 5931–5935 (1997).
Iizuka, K. et al. Requirement for membrane lymphotoxin in natural killer cell development. Proc. Natl Acad. Sci. USA 96, 6336–6340 (1999).
Elewaut, D. et al. Membrane lymphotoxin is required for the development of different subpopulations of NK T cells. J. Immunol. 165, 671–679 (2000).
Mombaerts, P. et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature 360, 225–231 (1992).
Dudley, E. C., Petrie, H. T., Shah, L. M., Owen, M. J. & Hayday, A. C. T cell receptor β chain gene rearrangement and selection during thymocyte development in adult mice. Immunity 1, 83–93 (1994).
Fehling, H. J., Krotkova, A., Saint-Ruf, C. & von Boehmer, H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature 375, 795–798 (1995).
MacDonald, H. R. CD1d-Glycolipid tetramers: A new tool to monitor natural killer T cells in health and disease. J. Exp. Med. 192, 15–19 (2000).
MacDonald, H. R., Lees, R. K. & Held, W. Developmentally regulated extinction of Ly-49 receptor expression permits maturation and selection of NK1.1+ T cells. J. Exp. Med. 187, 2109–2114 (1998).
Page, D. M., Kane, L. P., Allison, J. P. & Hedrick, S. M. Two signals are required for negative selection of CD4+CD8+ thymocytes. J. Immunol. 151, 1868–1880 (1993).
Hogquist, K. A. et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity 6, 389–399 (1997).
Sant'Angelo, D. B. et al. A molecular map of T cell development. Immunity 9, 179–186 (1998).
Lucas, B., Stefanova, I., Yasutomo, K., Dautigny, N. & Germain, R. N. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity 10, 367–376 (1999).
Hogquist, K. A. Assays of thymic selection. Fetal thymus organ culture and in vitro thymocyte dulling assay. Meth. Mol. Biol. 156, 219–232 (2001).
McGargill, M. A., Derbinski, J. M. & Hogquist, K. A. Receptor editing in developing T cells. Nature Immunol. 1, 336–341 (2000).
Swat, W., Dessing, M., Baron, A., Kisielow, P. & von Boehmer, H. Phenotypic changes accompanying positive selection of CD4+CD8+ thymocytes. Eur. J. Immunol. 22, 2367–2372 (1992).
Apostolou, I. et al. Murine natural killer T (NKT) cells contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl Acad. Sci. USA 96, 5141–5146 (1999).
Makino, Y. et al. Extrathymic development of Vα14+ T cells. J. Exp. Med. 177, 1399–1408 (1993).
Sato, K. et al. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J. Exp. Med. 182, 759–767 (1995).
Makino, Y., Kanno, R., Koseki, H. & Taniguchi, M. Development of Vα14+ NK T cells in the early stages of embryogenesis. Proc. Natl Acad. Sci. USA 93, 6516–6520 (1996).
Shimizu, T. et al. The majority of lymphocytes in the bone marrow, thymus and extrathymic T cells in the liver are generated in situ from their own preexisting precursors. Microbiol. Immunol. 43, 595–608 (1999).
Shimamura, M., Ohteki, T., Launois, P., Garcia, A. M. & MacDonald, H. R. Thymus-independent generation of NK1+ T cells in vitro from fetal liver precursors. J. Immunol. 158, 3682–3689 (1997).
Tilloy, F., Di Santo, J. P., Bendelac, A. & Lantz, O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur. J. Immunol. 29, 3313–3318 (1999).
Shores, E. W., Sharrow, S. O. & Singer, A. Presence of CD4 and CD8 determinants on CD4−CD8− murine thymocytes: passive acquisition of CD8 accessory molecules. Eur. J. Immunol. 21, 973–977 (1991).
Michie, A. M., Carlyle, J. R. & Zuniga-Pflucker, J. C. Early intrathymic precursor cells acquire a CD4low phenotype. J. Immunol. 160, 1735–1741 (1998).
Asarnow, D. M., Cado, D. & Raulet, D. H. Selection is not required to produce invariant T-cell receptor γ-gene junctional sequences. Nature 362, 158–160 (1993).
Baldwin, K. K., Trenchak, B. P., Altman, J. D. & Davis, M. M. Negative selection of T cells occurs throughout thymic development. J. Immunol. 163, 689–698 (1999).
Bendelac, A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182, 2091–2096 (1995).
Takahama, Y., Kosugi, A. & Singer, A. Phenotype, ontogeny, and repertoire of CD4−CD8− T cell receptor αβ+ thymocytes. Variable influence of self-antigens on T cell receptor Vβ usage. J. Immunol. 146, 1134–1141 (1991).
Wu, L., Pearse, M., Egerton, M., Petrie, H. & Scollay, R. CD4−CD8− thymocytes that express the T cell receptor may have previously expressed CD8. Int. Immunol. 2, 51–56 (1990).
Bendelac, A., Hunziker, R. D. & Lantz, O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184, 1285–1293 (1996).
Bruno, L., Fehling, H. J. & von Boehmer, H. The αβ T cell receptor can replace the γδ receptor in the development of γδ lineage cells. Immunity 5, 343–352 (1996).
Terrence, K., Pavlovich, C. P., Matechak, E. O. & Fowlkes, B. J. Premature expression of T cell receptor (TCR)αβ supresses TCRγδ gene rearrangement but permits development of γδ lineage T cells. J. Exp. Med. 192, 537–548 (2000).
Curnow, S. J., Boyer, C., Buferne, M. & Schmitt-Verhulst, A. M. TCR-associated ζ-FcɛRIγ heterodimers on CD4−CD8− NK1.1+ T cells selected by specific class I MHC antigen. Immunity 3, 427–438 (1995).
Schulz, R. J., Parkes, A., Mizoguchi, E., Bhan, A. K. & Koyasu, S. Development of CD4−CD8− αβTCR+NK1.1+ T lymphocytes: thymic selection by self antigen. J. Immunol. 157, 4379–4389 (1996).
Iwabuchi, C. et al. Intrathymic selection of NK1.1+α/β T cell antigen receptor (TCR)+ cells in transgenic mice bearing TCR specific for chicken ovalbumin and restricted to I-Ad. Proc. Natl Acad. Sci. USA 95, 8199–8204 (1998).
Legendre, V. et al. Selection of phenotypically distinct NK1.1+ T cells upon antigen expression in the thymus or in the liver. Eur. J. Immunol. 29, 2330–2343 (1999).
Sato, H. et al. Induction of differentiation of pre-NKT cells to mature Vα14 NKT cells by granulocyte/macrophage colony-stimulating factor. Proc. Natl Acad. Sci. USA 96, 7439–7444 (1999).
Iwabuchi, K. et al. Defective development of NK1.1+ T-cell antigen receptor αβ+ cells in zeta-associated protein 70 null mice with an accumulation of NK1.1+ CD3− NK-like cells in the thymus. Blood 97, 1765–1775 (2001).
Lantz, O., Sharara, L. I., Tilloy, F., Andersson, A. & DiSanto, J. P. Lineage relationships and differentiation of natural killer (NK) T cells: intrathymic selection and interleukin (IL)-4 production in the absence of NKR-P1 and Ly49 molecules. J. Exp. Med. 185, 1395–1401 (1997).
Amsen, D. & Kruisbeek, A. M. Thymocyte selection: not by TCR alone. Immunol. Rev. 165, 209–229 (1998).
Bendelac, A., Matzinger, P., Seder, R. A., Paul, W. E. & Schwartz, R. H. Activation events during thymic selection. J. Exp. Med. 175, 731–742 (1992).
Arase, H., Arase, N., Nakagawa, K., Good, R. A. & Onoe, K. NK1.1+ CD4+ CD8− thymocytes with specific lymphokine secretion. Eur. J. Immunol. 23, 307–310 (1993).
Leite-de-Moraes, M. C. et al. MHC class I-selected CD4−CD8−TCR-αβ+ T cells are a potential source of IL-4 during primary immune response. J. Immunol. 155, 4544–4550 (1995).
Chen, Y. H., Chiu, N. M., Mandal, M., Wang, N. & Wang, C. R. Impaired NK1+ T cell development and early IL-4 production in CD1- deficient mice. Immunity 6, 459–467 (1997).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278, 1623–1626 (1997).
Acknowledgements
We thank C. Lena (FACS facility at LIAI) and A. Saluk (FACS facility at TSRI) for help with cell sorting; K.Warren for help with spectratyping; O. Naidenko and S. Sidobre for providing the α-GalCer–CD1d tetramer; and S. Hedrick, F. Koning and A. Attinger for critical review of the manuscript. Supported by grants from the National Institutes of Health (RO1 CA52511), the Human Frontiers of Science Organization (to M. K.) and the Cancer Research Institute (to L. G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gapin, L., Matsuda, J., Surh, C. et al. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol 2, 971–978 (2001). https://doi.org/10.1038/ni710
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni710
This article is cited by
-
Exploiting autophagy balance in T and NK cells as a new strategy to implement adoptive cell therapies
Molecular Cancer (2023)
-
Intrinsic factors and CD1d1 but not CD1d2 expression levels control invariant natural killer T cell subset differentiation
Nature Communications (2023)
-
Markers and makers of NKT17 cells
Experimental & Molecular Medicine (2023)
-
The phosphatidylinositol-transfer protein Nir3 promotes PI(4,5)P2 replenishment in response to TCR signaling during T cell development and survival
Nature Immunology (2023)
-
Tnpo3 controls splicing of the pre-mRNA encoding the canonical TCR α chain of iNKT cells
Nature Communications (2023)