Abstract
CD1-restricted T cells can be activated by diverse lipids derived from mammals, bacteria and protozoa. Certain lipids function as antigens, which bind to CD1 proteins and contact T cell antigen receptors. Other lipids activate CD1-restricted T cells by functioning as adjuvants. By stimulating Toll-like receptors on antigen-presenting cells, these adjuvants alter cytokine secretion, lipid antigen synthesis and CD1 protein translation. Delineation of the separate mechanisms by which adjuvants and antigens activate CD1-restricted T cells is leading to new hypotheses about the functions of individual CD1 proteins during the transition from innate to acquired immune responses.
Similar content being viewed by others
Main
The realization that T cells can be activated by CD1 proteins presenting cellular lipids has opened new avenues of investigation into the types of molecules recognized by αβ T cell antigen receptors (TCRs). The antigen-presenting function of CD1 proteins was discovered by studies of unusual, long-chain fatty acids that are expressed in a few species related to mycobacteria1,2. Subsequent studies have shown that CD1-restricted T cells influence the outcomes of infection with bacteria, viruses, fungi and protozoa through the recognition of diacylglycerols3,4,5,6, acylated carbohydrates7,8, polyketides9,10, sphingolipids11,12, phospholipids13, lipopeptides14 and other molecules. Also, T cells can be activated by self lipids such as gangliosides15,16, sulfatides17, phosphatidylinositols18, phosphatidylethanolamines19 and phosphatidylcholines20 (Fig. 1). The unexpected molecular diversity of these T cell stimulants supports the hypothesis that CD1 proteins normally function in 'reporting' changes in the global lipid content of cells.
Because of increasing evidence that CD1 proteins are involved in the outcome of infectious and autoimmune disease, considerable energy is now devoted to understanding the precise functions of CD1-restricted T cells in immune regulation. Certain CD1 proteins are constitutively expressed on many antigen-presenting cells (APCs), and CD1d-restricted natural killer T (NKT) cells are 'famous' for their ability to circulate in a preprimed state that allows them to rapidly secrete cytokines. Therefore, most models propose that CD1-restricted T cells, especially CD1d-restricted NKT cells, act as 'gatekeepers' during the earliest stages of the innate immune response, controlling subsequent dendritic cell (DC) maturation, NK cell activation and major histocompatibility complex (MHC)–restricted T cell polarization.
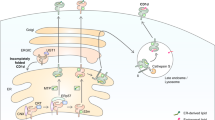
That view remains valid, but new discoveries indicate that it is incomplete. Toll-like receptors (TLRs) have been found to modulate the functions of CD1-restricted T cells through at least three distinct mechanisms (Fig. 2). CD1-restricted T cell activation can be influenced by TLR stimulation that occurs simultaneously with CD1-restricted T cell activation (Fig. 2b,c), as well as by TLR activation that occurs days before CD1 expression (Fig. 2d). Thus, certain aspects of CD1 function seem to be delayed and regulated by the innate immune system.
(a) Lipid antigens can be inserted into the groove of CD1 proteins and activate CD1-restricted T cells by direct contact with variable regions of TCRα and TCRβ chains. (b–d) Alternatively, lipids or other TLR agonists can stimulate APCs to secrete cytokines (b), to synthesize endogenous lipid antigens (c) or to translate new CD1 proteins (d). β-hex, β-hexosaminidase; LP, lipoprotein; LAM, lipoarabinomannan; PIM, phosphatidylinositol mannoside; ER, endoplasmic reticulum.
Also, the dynamic patterns of CD1 expression over time in infected cells or inflamed tissues are more complex than previously appreciated. Human APCs express five CD1 proteins which have been classified as belonging either to group 1 (CD1a, CD1b, CD1c and CD1e) or group 2 (CD1d)21. CD1d is constitutively expressed and therefore better fits the function of an innate receptor. In contrast, myeloid precursors of human DCs lack group 1 CD1 expression and express group 1 CD1 molecules only after activation by TLR ligands, cytokines or other stimuli. More generally, a picture is emerging in which APCs actively modulate transcription of genes encoding individual CD1 isoforms based on the local inflammatory milieu (Fig. 3). This review outlines new insights into the regulation of the formation of complexes between CD1 and lipid antigens in cells and emphasizes studies investigating the influence of TLRs on the antigen-presenting functions of CD1 proteins.
(a) Surface protein expression. CD1d is constitutively expressed on monocyte precursors, but such expression is influenced by local stimuli. In contrast, the transition from monocyte to immature DC involves a 'nothing-to-all' change in the expression of group 1 CD1 on the surface. Whereas MHC class II (MHC II) surface expression is substantially regulated by redirected trafficking of preformed proteins to the cell surface, increased group 1 protein expression occurs through new protein translation. This idealized model and most studies of the kinetics of CD1 expression are based on activation with high doses of granulocyte-macrophage colony-stimulating factor and recombinant IL-4 (GM-CSF + IL-4), but other studies have investigated the weaker but natural stimuli that may regulate CD1 expression in vivo. PPAR-γ, peroxisome proliferator activator receptor- γ; HIV, human immunodeficiency virus; KSHV, Kaposi sarcoma–associated herpesvirus. (b) T cell activation. The capacity of DCs to activate MHC class II–restricted T cells is regulated by functional transitions involving acquisition of antigen uptake as well as expression of costimulatory molecules72,80,81. Therefore, cell surface density of MHC class II does not always correlate with the ability to prime T cells. The acquisition of CD1-mediated T cell–activating properties occurs early in DC differentiation, and for group 1 CD1 expression, it may correlate in time with the first appearance of these proteins on the cell surface.
TCR recognition of lipid antigens
Before the discovery of the CD1 system, there was little precedent for the finding that either B cell antigen receptors or TCRs recognize molecules composed mainly of alkyl chains. Crystal structures of CD1a, CD1b and CD1d proteins in complex with lipid ligands have clarified the molecular basis by which hydrophobic and flexible molecules are presented to T cells22,23,24,25,26,27,28,29. CD1 proteins have an MHC-like fold that produces unusually deep antigen-binding grooves with up to four pockets, called A′, C′, T′ and F′ (refs. 22,23). Alkyl chains of lipid antigens are sequestered from aqueous solvent in the α1-α2 'superdomain' of CD1 proteins. The 'seating' of the most hydrophobic elements in the groove allows the inorganic, carbohydrate or peptidic components of antigens to protrude to the outer face of CD1 proteins for contact with TCRs (Fig. 2a).
Ternary CD1-lipid-TCR structures have not yet been captured by X-ray crystallography. However, the function of the TCR in mediating lipid antigen recognition has been demonstrated in experiments in which the transfer of TCRα and TCRβ chains into cells expressing other components of the CD3 signaling complex conferred lipid antigen reactivity on the recipient cells30,31. Furthermore, the direct interaction of TCRs with CD1-lipid complexes has been seen in binding studies32,33 and by the ability of lipid-loaded, fluorescent CD1 tetramers to stain T cells34,35,36. Through modeling studies27 and by the generation of point mutations in the gene sequence encoding in complementarity-determining region 3 of TCRs37 and in the α-helical regions of CD1 (ref. 38), detailed hypotheses about the potential 'footprint' of the TCR on the surface of CD1 have emerged. Crystal structures show that the overall conformations of TCRα and TCRβ chains of α-galactosyl ceramide–specific TCRs are similar to those of peptide-specific TCRs33,39. Docking models predict that complementarity-determining region 3 areas of TCRα and TCRβ chains are likely to contact CD1 at the point from which the glycolipid protrudes from the groove27,33,37,39. Thus, the central molecular paradigm of lipid-mediated T cell activation involves a trimolecular CD1-lipid-TCR interaction, which is comparable to TCR recognition of cognate peptide-MHC complexes (Fig. 2a).
Formation of CD1-lipid complexes in cells
That model raises issues about how APCs select among the various types of lipid available for binding to CD1 and regulate the insertion of lipid antigens into the hydrophobic groove. Self lipid ligands can come from within the CD1-expressing APC, possibly during the cotranslational folding of CD1 proteins around lipids or by exchange reactions catalyzed by microsomal triglyceride transfer proteins during CD1 transit through the secretory pathway40,41. Once present at the cell surface, CD1 proteins have an ability to capture and present some types of exogenous lipid without the need for cellular cofactors15,18,42. Other lipids have more stringent loading requirements, such that they do not efficiently load onto cell surface CD1 proteins but instead selectively associate with CD1 proteins in endosomes1,43,44,45,46.
The dynamic processes by which antigens are inserted into the grooves of CD1 proteins are not yet understood, but studies have indicated certain molecular variables that influence loading at the cell surface or in acidic endosomes. In particular, lipids with short alkyl chains47 and several ligands for human CD1a42, which has a particularly shallow antigen-binding groove24, can be loaded at neutral pH on the cell surface. In contrast, lipids with longer alkyl chains tend to require endosomal loading1,8,48, and one study has indicated that long alkyl chain length may be the key variable necessitating transport of CD1 proteins and lipids to endosomes for loading reactions47.
The entrances to CD1 grooves are relatively narrow (9–17 Å) compared with those in MHC proteins (over 22 Å)22,24,33. Thus, antigens with the longest alkyl chains might experience more steric hindrance as they traverse the entrance to the groove. Likewise, lipids with long alkyl chains might encounter more loss of free energy of desolvation during transient exposure to aqueous solution, while they are transferred from membranes, lipid-protein complexes or other donor substrates into the groove. Therefore, the correlation of long chain length and large groove volume with the necessity for transport to endosomes might be explained if endosomes provide loading cofactors that overcome those steric and solvent hindrances.
Consistent with that hypothesis, endosomes serve as the site of accumulation of saposins and apolipoprotein E, which are lipid-transfer proteins that promote the delivery and insertion of lipids into the CD1 groove49,50,51,52. In addition, CD1e proteins function as lipid-binding proteins that assist in exposing glycolipids such as phosphatidylinositol mannoside (Fig. 1) to allow glycan trimming in endosomes before T cell activation6,53. Finally, the low pH of late endosomes and lysosomes (pH 4.5–5.5) partially and reversibly denatures the CD1 α-helices that protect the entrance to the groove54. Thus, CD1 proteins may function as pH-regulated lipid clamps, whose grooves transiently become more accessible as the proteins travel through late endosomes and lysosomes55.
By regulating which lipids enter late endosomes, APCs can control the types of lipid that are ultimately displayed on CD1 to T cells. The lumen of endosomal compartments is enriched in exogenously derived lipids, including those that are delivered by the action of regulated macropinocytosis56, mannose receptors57, langerin58 and the low-density lipoprotein receptor52. Galactosidase A (ref. 59) and other lysosomal glycosidases60 trim some glycans from these glycolipids, ultimately revealing carbohydrate epitopes, but there is no evidence so far that the alkyl chains of lipid antigens are trimmed to fit the grooves of CD1 proteins55.
After CD1-lipid complexes form in endosomes, they are exported to the surface for recognition by TCRs. The idea that lipids can act as cognate antigens by directly contacting the TCR no longer attracts controversy because it is simple and explains much experimental data (Fig. 2a). However, a decade ago, it was proposed on a theoretical basis that lipids might also activate CD1-restricted T cells by parallel pathways that do not solely involve lipid insertion into CD1 and contact with TCRs61. Now, data have shown that lipids do indeed activate CD1-restricted T cells through three distinct noncognate pathways and each of those pathways involves TLRs (Fig. 2b–d).
Pathway 1: lipid-induced cytokine synthesis
Infection by Gram-negative bacteria, including Salmonella typhimurium and Pseudomonas aeruginosa, activates CD1d-restricted NKT cells12,62, and in some cases, NKT cells contribute to the resolution of infection63. To identify factors mediating this response, Gram-negative bacterial cell walls were extracted with aqueous and organic solvents. Stimulatory factors were found to be protease resistant and were then identified as lipopolysaccharide (LPS) molecules64 (Fig. 1). Activation of NKT cells by LPS in vitro required that APCs express CD1d, but mechanistic experiments indicated that LPS might work by mechanisms other than direct interaction with the TCR.
LPS, a well known agonist of TLR4, stimulates secretion of interleukin 12 p70 (IL-12p70) from DCs, and interleukin 12 is sufficient to promote NKT cell activation in vitro in the absence of LPS, as long as APCs also express CD1d. In vivo, blockade of IL-12 reduces the number of interferon-γ-secreting NKT cells64. Those studies indicate that CD1d and foreign glycolipids may be able to activate T cells by sending two separate and synergistic signals transmitted through TCRs and TLRs, respectively (Fig. 2b).
Pathway 2: lipid-induced lipid synthesis
Building on evidence that CD1d proteins bind endogenous lipids as they recycle from the cell surface to late endosomes and lysosomes and back to the cell surface43,44,65,66,67, deletion of a lysosomal enzyme, β-hexosaminidase, was found to block positive selection of CD1d-restricted NKT cells60. Although deletion of glycosidases might nonspecifically inhibit lipid antigen presentation by globally altering the glycolipid content and the physical structure of lysosomes, β-hexosaminidase-deficient cells were found to be deficient in mediating T cell recognition of only certain glycolipids. In particular, β-hexosaminidase is known to convert isogloboside4 (iGb4) to isogloboside3 (iGb3). Both iGb3 and iGb4 stimulated NKT cells, but whereas iGb4-mediated T cell stimulation required β-hexosaminidase expression by APCs, iGb3 could be presented to T cells by recombinant CD1d proteins coated on plastic plates. Thus, it seems that iGb4 is a precursor and that iGb3, when bound to CD1d, can directly stimulate TCRs.
Whereas most previous studies have emphasized T cell stimulation by exogenous lipid antigens that are taken up into the endosomal network for loading into the grooves of CD1 proteins (Fig. 2a), these studies indicate that activated DCs can be triggered to produce an endogenous glycolipid antigen de novo (Fig. 2c). Separate studies searching for stimuli that might regulate this process showed that the β-hexosaminidase-dependent activation of NKT cells could be triggered by S. typhimurium in a way that depends on adaptor molecule MyD88 (ref. 12). Those results were interpreted to indicate that Gram-negative LPS may act through TLR4 to facilitate NKT cell activation and that this process may also requires β-hexosaminidase-mediated production of endogenous isoglobosides.
Certain interconnections among these key elements of the isogloboside pathway remain to be investigated, including the presumed function of an LPS-TLR4 interaction in this process and direct measurements of altered endogenous iGb3 production and the association of iGb3 with CD1d in intact cells. However, these studies have identified an unexpected mechanism for CD1-mediated T cell activation whereby an exogenous foreign glycolipid controls production of a structurally unrelated endogenous mammalian glycolipid, which in turn is likely to function as a cognate TCR antigen. This new idea is also likely to be relevant to the functions of group 1 CD1 isoforms. For example, studies have found that treating APCs with bacteria, mycobacteria or TLR agonists can promote the activation of CD1a- or CD1b-restricted T cell lines, even though these T cell lines express TCRs that are not specific for bacterial products but instead recognize self glycosphingolipids68. That finding was interpreted to indicate that microbial stimuli may alter endogenous mammalian lipid antigens that bind to CD1a and CD1b. In addition, TLR agonists might directly influence the functions of T cells, as NKT cells express TLR4 (ref. 69).
Pathway 3: regulation of CD1 protein synthesis
As with MHC class II, regulation of CD1 cell surface expression might be one mechanism by which APCs control responses to different classes of self and foreign lipids. Until recently, that possibility had not been widely investigated because CD1d, the most extensively studied of the mammalian CD1 isoforms, is constitutively expressed on marginal zone B cells, mouse bone marrow DCs and monocytic precursors of human DCs61 (Fig. 3). However, group 1 CD1 isoforms more clearly show inducible patterns of expression. Blood monocytes do not detectably express CD1a, CD1b or CD1c, but these proteins can be upregulated to high density on the cell surface by granulocyte-macrophage colony-stimulating factor, IL-4 and other cytokine 'cocktails' designed to drive DC differentiation in vitro1,70. The use of recombinant cytokines to engineer CD1-expressing cells gave rise to the idea that a similar process might occur in vivo and led to efforts to identify natural stimuli that regulate group 1 CD1 protein expression.
One possibility is that myeloid cells might come to express group 1 CD1 proteins as the result of contact with infecting pathogens or other localized inflammatory stimuli in tissues. Mycobacterium tuberculosis and Mycobacterium leprae, which produce cognate antigens that bind to CD1, also trigger the appearance of group 1 CD1 proteins on the surface of infected cells56,71. The factors inducing group 1 CD1 protein expression are shed from the surface of live mycobacteria and are enriched in the lipid-containing cell wall fractions. Several mycobacterial lipids are sufficient to upregulate group 1 CD1 expression in DCs, including two glycolipid agonists of TLR2: lipoarabinomannan and phosphatidylinositol mannoside56 (Fig. 1). Studies of DCs in human skin infected have shown that infection with M. leprae or treatment of cells with purified agonists of TLR5 and TLR2, including a 19-kDa lipoprotein, is sufficient to induce expression of group 1 CD1 proteins71.
Although the precise signaling mechanisms by which TLR activation leads to expression of group 1 CD1 proteins are not yet known, the cellular program involves cytokine secretion and new CD1 protein synthesis, a process that requires 2–3 days (refs. 1,55). CD1-restricted T cells are often considered innate lymphocytes that function during the first hours of an immune response. However, those studies have indicated that group 1 CD1 proteins on activated myeloid cells may be regulated by innate pattern-recognition receptors and may function at a later stage of immune response. The apparent conflict between these two points of view can be resolved by appreciating that CD1d and group 1 CD1 proteins differ in the stimuli, timing and cellular mechanisms of their expression on DCs (Fig. 3).
Regulated expression of CD1 on DCs
Although many types of APC express CD1 proteins, the function of CD1 on myeloid DCs has emerged as a central area of study. MHC class II, CD1d and group 1 CD1 proteins are expressed together at intermediate stages of DC differentiation, but it has been possible to delineate distinct differences in the kinetics and cellular mechanisms that control their cell surface expression (Fig. 3a). The efficiency of peptide antigen processing and presentation by MHC class II is modulated based on the types of activating stimulus encountered in the periphery. DCs gradually progress through a program of maturation to become competent APCs as they accrue antigen-processing and costimulatory functions in a stepwise way. As part of that process, the surface density of MHC class II proteins increases from moderate to high during relatively late stages of DC maturation as a result of redirected trafficking of peptide-MHC complexes from endosomes to the cell surface72.
In contrast to that intracellular redistributive phenomenon, the initial appearance of group 1 CD1 proteins on immature DCs results mainly or solely from new protein translation56. Monocytic precursors of DCs seem to lack group 1 CD1 transcripts and intracellular and surface proteins. After exposure to mycobacteria, cytokines, TLR agonists or related stimuli, kinetic analyses have shown parallel upregulation of mRNA encoding group 1 CD1 proteins and of its corresponding cell surface proteins over a period of about 72 h (Fig. 3a).
In contrast, CD1d is constitutively expressed on monocytes, macrophages and myeloid DCs at many stages of maturation. The absolute CD1d surface expression can be modulated from intermediate to high or low density by viruses73,74,75, bacteria56,76 and agonists of peroxisome proliferator activator receptor-γ77. For example, interferon-γ combined with M. tuberculosis infection or purified TLR2 agonists results in upregulation of CD1d expression on macrophages, such that the activation of NKT cells is augmented76. Conversely, Kaposi sarcoma–associated herpes virus downregulates surface expression of human CD1d through the action of MIR (modulator of immune recognition) proteins through a mechanism that involves ubiquitination of the CD1d cytoplasmic tail and redirected trafficking of CD1d to lysosomes73.
Not only do groups 1 and 2 CD1 proteins differ in their timing of expression on DCs, but also the transcription of genes encoding groups 1 and 2 CD1 proteins can be oppositely regulated by a single stimulus. For example, agonists of peroxisome proliferator activator receptor-γ induce expression of the CD1d gene and protein while simultaneously suppressing expression of the gene encoding CD1a77. Similarly, mycobacterial lipids upregulate transcripts encoding group 1 CD1 molecules and downregulate those encoding CD1d in infected or activated cells55. Thus, whereas CD1 proteins were originally classified into two separate groups based solely on sequence homology21, those studies of early and late gene expression in DCs are emerging as a second general criterion that distinguishes group 1 versus group 2 CD1 proteins. Given the potential importance of this phenomenon, it is surprising that 20 years after the discovery of the CD1 locus, little detailed mechanistic information exists about potential CD1 isoform-specific promoters and transcription factors78. E26 transformation–specific transcription factors have been shown to regulate the expression of CD1d (ref. 79).
Functional transitions in DC differentiation
Studies now seek to move beyond quantitative measurement of CD1 protein density on the cell surface to focus on the stimuli that actually control the ability of APCs to activate or prime CD1-restricted T cells (Fig. 3b). For MHC class II–mediated peptide antigen presentation, the functionally important transitions in DC differentiation are well studied. Quiescent myeloid cells first acquire the ability to rapidly internalize exogenous antigens and cleave them into peptides as they develop into immature DCs. Subsequently, the transition from immature to mature DCs involves release of peptide-MHC complexes from endosomes to the surface, expression of costimulatory molecules and other changes, all of which are critical for controlling whether the TCR stimulation results in T cell priming or anergy72,80,81. Thus, two functionally important transitions in DC maturation regulate the stimulation of MHC class II–restricted CD4+ T cells, and the amount of MHC class II surface expression (Fig. 3a) does not fully correlate with T cell stimulatory capacity (Fig. 3b).
Diagrams of changes in the T cell–activating capacity of CD1-expressing myeloid cells are incomplete (Fig. 3b), but hypotheses about the physiological factors that regulate the CD1-mediated T cell–activating capacity are emerging. Cell surface expression of CD1 is necessary for antigen display, but other cellular factors influence the efficiency of T cell activation. For example, NKT cell activation by vesicular stomatitis virus and vaccinia virus can be substantially inhibited without changing CD1d surface expression82. Conversely, when expressed in an otherwise competent APC, a low surface density of CD1d proteins is sufficient to efficiently activate NKT cells83. The discordance between cell surface density of CD1 and the efficiency of T cell activation probably relates to factors such as rates of antigen uptake, availability of endosomal lipid transfer proteins and rates of recycling of CD1 proteins to endosomes. Therefore, it will be useful to further investigate whether these factors are concurrently modulated with CD1 protein expression during DC maturation.
Of the three antigen-presenting systems discussed here, group 1 CD1 proteins are the only ones absent within and on the surface of myeloid precursors of DCs. Given the 'all-or-nothing' nature of the changes in group 1 CD1 expression that occur during the transition of monocytes to immature DCs, it is plausible that regulated CD1 transcription and translation is a central means for regulating the functions of group 1 CD1–restricted T cells. In vitro, the cellular machinery necessary for antigen processing and display, even for certain antigens that undergo stringent loading reactions in endosomes, is acquired during the early phases of DC differentiation1,8,47,56. In addition, one study has suggested that later DC maturation events do not further enhance the ability of DCs to stimulate CD1-restricted T cell lines84. Although the factors that govern priming of group 1 CD1–restricted T cells in vivo are not known, immature DCs are able to support the proliferation of polyclonal human T cells ex vivo8,9,85. These studies support the hypothesis that group 1 CD1 antigen presentation systems undergo a functional transition during an early stage of DC differentiation and that this transition may be more tightly correlated with CD1 protein synthesis than transitions regulating the function of MHC class II antigen presentation systems (Fig. 3b).
Because CD1d is constitutively expressed on myeloid precursors of DCs and CD1d-restricted NKT cells are 'preprimed'86, it is less clear that CD1d antigen presentation systems necessarily undergo any similar functional transitions during DC differentiation. Instead, TLRs and CD1d probably function simultaneously at the earliest stages of the immune response. One model emphasizes the intertwined nature of CD1d and TLR signals by showing how TLR stimulation provides IL-12- or isogloboside–mediated signals that also require CD1d-TCR interactions59,64 (Fig. 2b,c). A different model proposes an 'either/or' scheme in which certain pathogens supply ligands for TLRs and other pathogens supply ligands for CD1. The last model derives from the observation that CD1d-presented α-glucuronyl ceramides (Fig. 1) are found in certain types of Gram-negative bacteria that naturally lack LPS agonists for TLR4 (refs. 11,12). In this model, CD1d and TLR4 are not viewed as synergistic, but instead are considered as acting separately to accomplish similar functions in response to a broader range of pathogens.
Immunogenic lipid packets
Whereas these models of CD1d function emphasize rapid T cell activation, the initial lack of group 1 CD1 molecules on peripheral monocytes supports a two-step model whereby TLR activation precedes and controls group 1 CD1 expression, thereby acting as a 'gateway' to CD1 function. Such a system might localize group 1 CD1 lipid antigen presentation in time and space to sites of infection. That hypothesis is supported by the observation that cognate lipid antigens are more efficiently presented when administered with TLR2 ligands or in the form of intact bacterial cell walls14,56. Although it is not possible at present to directly track the upregulation of group1 CD1 proteins in human tissues over time, myeloid cells expressing group 1 CD1 proteins are present in the skin of patients with tuberculoid leprosy71,87 and broncheoalveolar lavage samples from patients with tuberculosis88. The CD1+ cells appear at sites of infection in vivo, either through upregulation of CD1 in situ or migration of CD1-expressing cells.
Lipids normally self assemble in aqueous biological solutions to form mixed aggregates described as micelles, membranes and lipid-protein complexes. Thus, bilayers containing lipid antigens may normally coat or otherwise be physically associated with lipid, protein or nucleic acid agonists of TLRs. For example, mycobacterial cell walls contain lipid TLR agonists (phosphatidylinositol mannoside, lipoarabinomannan and 19-kDa lipoprotein) intercalated with lipid antigens (mycolic acid, glucose monomycolate and others). More generally, antigens presented by CD1 molecules are efficiently provided to APCs as membrane fragments, apoptotic bodies89 or highly ordered lipoprotein complexes52. Thus, microbes may provide both the 'on switch' (TLR agonists) to stimulate exogenous lipid processing and the raw material (lipid antigens) that is ultimately loaded into CD1 proteins.
Distinguishing antigens from adjuvants
Not all lipids that stimulate CD1-restricted T cells are antigens (Fig. 1). If the term 'antigen' is restricted to only those molecules that directly bind to TCRs (Fig. 2a), then lipids that stimulate APCs to produce cytokines, endogenous lipids and CD1 proteins represent adjuvants90 (Fig. 2b–d). LPS is an adjuvant12,59,64, whereas α-galactosyl ceramide is an antigen32,33. Other lipids such as phosphatidylinositol mannoside and lipoarabinomannan have been proposed to have both adjuvant and antigenic properties, so that both functions may be present in a single molecule3,6,56. The dominant mechanism of action for these and other lipids remains unknown, so it is possible that lipids previously described as 'antigens' may actually function as adjuvants.
Experimentally distinguishing between antigen and adjuvant functions is conceptually important and may offer practical advantages for therapy. Based on new insights into the function of CD1-restricted T cells in asthma91 and other diseases, lipid-mediated activation, deactivation or polarization of T cells might be used to treat diseases that involve T cells. Approaches now in use administer lipid antigens directly, preload antigens on DCs92,93 or provide chemically altered antigens that skew the T helper type 1 versus T helper type 2 cytokine balance94,95. Understanding whether any given lipid works directly as a cognate antigen or indirectly through the APC is fundamental to the design of such therapies. For example, it might be possible to administer lipid antigens together with CD1 adjuvants to produce optimal therapeutic effects. Studies showing the differing effects of TLR ligands, peroxisome proliferator activator receptor-γ agonists and other adjuvants on MHC class II, CD1d and group 1 CD1 pathways indicate that antigens may need to be paired carefully with the proper type of adjuvant56,77, depending on whether the goal is to activate NKT cells, the diverse T cells restricted by group 1 CD1 isoforms or CD1-restricted T cells that influence MHC-restricted T cells93,96 (Fig. 3). Thus, a more detailed understanding of the molecular pathways by which TLR gateways naturally control CD1 function could pave the way for lipid antigen–specific T cell immunomodulation.
References
Porcelli, S., Morita, C.T. & Brenner, M.B. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature 360, 593–597 (1992).
Beckman, E.M. et al. Recognition of a lipid antigen by CD1-restricted αβ T cells. Nature 372, 691–694 (1994).
Sieling, P.A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Schofield, L. et al. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283, 225–229 (1999).
Fischer, K. et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA 101, 10685–10690 (2004).
de la Salle, H. et al. Assistance of microbial glycolipid antigen processing by CD1e. Science 310, 1321–1324 (2005).
Moody, D.B. et al. Structural requirements for glycolipid antigen recognition by CD1b- restricted T cells. Science 278, 283–286 (1997).
Gilleron, M. et al. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199, 649–659 (2004).
Moody, D.B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Matsunaga, I. et al. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J. Exp. Med. 200, 1559–1569 (2004).
Kinjo, Y. et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Amprey, J.L. et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200, 895–904 (2004).
Moody, D.B. et al. T cell activation by lipopeptide antigens. Science 303, 527–531 (2004).
Shamshiev, A. et al. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29, 1667–1675 (1999).
Wu, D.Y., Segal, N.H., Sidobre, S., Kronenberg, M. & Chapman, P.B. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198, 173–181 (2003).
Shamshiev, A. et al. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 195, 1013–1021 (2002).
Gumperz, J. et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Rauch, J. et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J. Biol. Chem. 278, 47508–47515 (2003).
Agea, E. et al. Human CD1-restricted T cell recognition of lipids from pollens. J. Exp. Med. 202, 295–308 (2005).
Calabi, F., Jarvis, J.M., Martin, L. & Milstein, C. Two classes of CD1 genes. Eur. J. Immunol. 19, 285–292 (1989).
Zeng, Z. et al. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277, 339–345 (1997).
Gadola, S.D. et al. Structure of human CD1b with bound ligands at 2.3 Å, a maze for alkyl chains. Nat. Immunol. 3, 721–726 (2002).
Zajonc, D.M., Elsliger, M.A., Teyton, L. & Wilson, I.A. Crystal structure of CD1a in complex with a sulfatide self antigen at a resolution of 2.15 Å. Nat. Immunol. 4, 808–815 (2003).
Batuwangala, T. et al. The crystal structure of human CD1b with a bound bacterial glycolipid. J. Immunol. 172, 2382–2388 (2004).
Zajonc, D.M. et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat. Immunol. 6, 810–818 (2005).
Zajonc, D.M. et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22, 209–219 (2005).
Koch, M. et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat. Immunol. 6, 819–826 (2005).
Giabbai, B. et al. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: a molecular basis for NKT cell activation. J. Immunol. 175, 977–984 (2005).
Kawano, T. et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 (1997).
Grant, E.P. et al. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 189, 195–205 (1999).
Sidobre, S. et al. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 169, 1340–1348 (2002).
Gadola, S.D. et al. Structure and binding kinetics of three different human CD1d–α-galactosylceramide–specific T cell receptors. J. Exp. Med. 203, 699–710 (2006).
Matsuda, J.L. et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192, 741–754 (2000).
Benlagha, K., Weiss, A., Beavis, A., Teyton, L. & Bendelac, A. In vivo identification of glycolipid antigen–specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191, 1895–1903 (2000).
Karadimitris, A. et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl. Acad. Sci. USA 98, 3294–3298 (2001).
Grant, E.P. et al. Fine specificity of TCR complementarity-determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1-lipid complex. J. Immunol. 168, 3933–3940 (2002).
Melian, A. et al. Molecular recognition of human CD1b antigen complexes: evidence for a common pattern of interaction with αβ TCRs. J. Immunol. 165, 4494–4504 (2000).
Kjer-Nielsen, L. et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J. Exp. Med. 203, 661–673 (2006).
De Silva, A.D. et al. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J. Immunol. 168, 723–733 (2002).
Brozovic, S. et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat. Med. 10, 535–539 (2004).
Manolova, V. et al. Functional CD1a is stabilized by exogenous lipids. Eur. J. Immunol. 36, 1083–1092 (2006).
Chiu, Y.H. et al. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail–truncated CD1d. Nat. Immunol. 3, 55–60 (2002).
Chiu, Y.H. et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189, 103–110 (1999).
Jackman, R.M. et al. The tyrosine-containing cytoplasmic tail of CD1b is essential for its efficient presentation of bacterial lipid antigens. Immunity 8, 341–351 (1998).
Roberts, T.J. et al. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J. Immunol. 168, 5409–5414 (2002).
Moody, D.B. et al. Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat. Immunol. 3, 435–442 (2002).
Moody, D.B., Reinhold, B.B., Reinhold, V.N., Besra, G.S. & Porcelli, S.A. Uptake and processing of glycosylated mycolates for presentation to CD1b-restricted T cells. Immunol. Lett. 65, 85–91 (1999).
Winau, F. et al. Saposin C is required for lipid presentation by human CD1b. Nat. Immunol. 5, 169–174 (2004).
Kang, S.J. & Cresswell, P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat. Immunol. 5, 175–181 (2004).
Zhou, D. et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science 303, 523–527 (2004).
van den Elzen, P. et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005).
Angenieux, C. et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J. Biol. Chem. 275, 37757–37764 (2000).
Ernst, W.A. et al. Molecular interaction of CD1b with lipoglycan antigens. Immunity 8, 331–340 (1998).
Cheng, T. et al. Role of lipid trimming, acid pH and CD1 groove size in presenting lipid antigens. EMBO J. advance online publication 22 June 2006 (doi: 10.1038/sj.emboj.7601185).
Roura-Mir, C. et al. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J. Immunol. 175, 1758–1766 (2005).
Prigozy, T.I. et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6, 187–197 (1997).
Hunger, R.E. et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J. Clin. Invest. 113, 701–708 (2004).
Prigozy, T.I. et al. Glycolipid antigen processing for presentation by CD1d molecules. Science 291, 664–667 (2001).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Porcelli, S.A. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59, 1–98 (1995).
Berntman, E., Rolf, J., Johansson, C., Anderson, P. & Cardell, S.L. The role of CD1d-restricted NK T lymphocytes in the immune response to oral infection with Salmonella typhimurium. Eur. J. Immunol. 35, 2100–2109 (2005).
Nieuwenhuis, E.E. et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 8, 588–593 (2002).
Brigl, M., Bry, L., Kent, S.C., Gumperz, J.E. & Brenner, M.B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Jayawardena-Wolf, J., Benlagha, K., Chiu, Y.H., Mehr, R. & Bendelac, A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity 15, 897–908 (2001).
Elewaut, D. et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Vα14i NKT cells. J. Exp. Med. 198, 1133–1146 (2003).
Cernadas, M. et al. Lysosomal localization of murine CD1d mediated by AP-3 is necessary for NK T cell development. J. Immunol. 171, 4149–4155 (2003).
De Libero, G. et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity 22, 763–772 (2005).
Askenase, P.W. et al. TLR-dependent IL-4 production by invariant Vα14+Jα18+ NKT cells to initiate contact sensitivity in vivo. J. Immunol. 175, 6390–6401 (2005).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony- stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Krutzik, S.R. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11, 653–660 (2005).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Sanchez, D.J., Gumperz, J.E. & Ganem, D. Regulation of CD1d expression and function by a herpesvirus infection. J. Clin. Invest. 115, 1369–1378 (2005).
Chen, N. et al. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 36, 278–286 (2006).
Cho, S. et al. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 337, 242–252 (2005).
Skold, M., Xiong, X., Illarionov, P.A., Besra, G.S. & Behar, S.M. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J. Immunol. 175, 3584–3593 (2005).
Szatmari, I. et al. Activation of PPARγ specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity 21, 95–106 (2004).
Calabi, F. & Milstein, C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature 323, 540–543 (1986).
Geng, Y., Laslo, P., Barton, K. & Wang, C.R. Transcriptional regulation of CD1D1 by Ets family transcription factors. J. Immunol. 175, 1022–1029 (2005).
Trombetta, E.S., Ebersold, M., Garrett, W., Pypaert, M. & Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 (2003).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Renukaradhya, G.J. et al. Virus-induced inhibition of CD1d1-mediated antigen presentation: reciprocal regulation by p38 and ERK. J. Immunol. 175, 4301–4308 (2005).
Spada, F.M. et al. Low expression level but potent antigen presenting function of CD1d on monocyte lineage cells. Eur. J. Immunol. 30, 3468–3477 (2000).
Cao, X. et al. CD1 molecules efficiently present antigen in immature dendritic cells and traffic independently of MHC class II during dendritic cell maturation. J. Immunol. 169, 4770–4777 (2002).
Ulrichs, T., Moody, D.B., Grant, E., Kaufmann, S.H. & Porcelli, S.A. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect. Immun. 71, 3076–3087 (2003).
Bendelac, A. & Medzhitov, R. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J. Exp. Med. 195, F19–F23 (2002).
Sieling, P.A. et al. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J. Immunol. 162, 1851–1858 (1999).
Buettner, M. et al. Inverse correlation of maturity and antibacterial activity in human dendritic cells. J. Immunol. 174, 4203–4209 (2005).
Schaible, U.E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1046 (2003).
Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54 Pt. 1, 1–13 (1989).
Akbari, O. et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 354, 1117–1129 (2006).
Fujii, S., Shimizu, K., Kronenberg, M. & Steinman, R.M. Prolonged IFN-γ–producing NKT response induced with α-galactosylceramide–loaded DCs. Nat. Immunol. 3, 867–874 (2002).
Silk, J.D. et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Invest. 114, 1800–1811 (2004).
Miyamoto, K., Miyake, S. & Yamamura, T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413, 531–534 (2001).
Yu, K.O. et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl. Acad. Sci. USA 102, 3383–3388 (2005).
Vincent, M.S. et al. CD1-dependent dendritic cell instruction. Nat. Immunol. 3, 1163–1168 (2002).
Acknowledgements
I thank C. Roura-Mir, L. Wang, D. Young, K. Wucherpfennig, S. Behar, M. Brigl and V. Cerundolo for discussions and for providing data for figures. Supported by the Cancer Research Institute, the Pew Foundation Scholars in the Biomedical Sciences and the National Institutes of Health (AI071155, AR48632 and AI049313).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Moody, D. TLR gateways to CD1 function. Nat Immunol 7, 811–817 (2006). https://doi.org/10.1038/ni1368
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1368
This article is cited by
-
Glycolipid iGb3 feedback amplifies innate immune responses via CD1d reverse signaling
Cell Research (2019)
-
Delineating the autoimmune mechanisms in Graves’ disease
Immunologic Research (2012)
-
Mycobacterium tuberculosis impairs dendritic cell response by altering CD1b, DC‐SIGN and MR profile
Immunology & Cell Biology (2010)
-
Dendritic Cells and Toll-Like Receptors 2 and 4 in the Ileum of Crohn's Disease Patients
Digestive Diseases and Sciences (2008)