Abstract
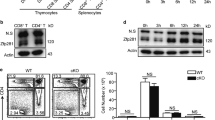
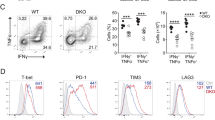
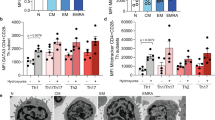
Gadd45β (growth arrest and DNA damage–inducible, β) is involved in cell cycle arrest, apoptosis, signal transduction and cell survival. In T cells, Gadd45b was rapidly induced by T cell receptor (TCR) and inflammatory signals. Deficiency of Gadd45β in CD4+ T cells impaired their responses to TCR stimulation or inflammatory cytokines. ERK, p38 and JNK activation were all substantially suppressed in Gadd45β-deficient CD4+ T cells. Cytokine production by Gadd45β-deficient CD4+ T cells was also impaired. Furthermore, Gadd45β mediated inflammatory cytokine production by dendritic cells, and Gadd45β-deficient mice showed an impaired T helper type 1 response during Listeria monocytogenes infection. Gadd45β is therefore a critical feedback regulator that perpetuates both cognate and inflammatory signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Liebermann, D.A. & Hoffman, B. Myeloid differentiation (MyD) primary response genes in hematopoiesis. Oncogene 21, 3391–3402 (2002).
Hollander, M.C. & Fornace, A.J. Jr. Genomic instability, centrosome amplification, cell cycle checkpoints and Gadd45α. Oncogene 21, 6228–6233 (2002).
Chakravarty, D. et al. Three GADD45 isoforms contribute to hypertonic stress phenotype of murine renal inner medullary cells. Am. J. Physiol. Renal Physiol. 283, F1020–1029 (2002).
Vairapandi, M., Balliet, A.G., Hoffman, B. & Liebermann, D.A. GADD45β and GADD45γ are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell Physiol. 192, 327–338 (2002).
Yang, J., Zhu, H., Murphy, T.L., Ouyang, W. & Murphy, K.M. IL-18-stimulated GADD45β required in cytokine-induced, but not TCR-induced, IFN-γ production. Nat. Immunol. 2, 157–164 (2001).
Lu, B. et al. GADD45γ mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity 14, 583–590 (2001).
De Smaele, E. et al. Induction of gadd45b by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414, 308–313 (2001).
Takekawa, M. et al. Smad-dependent GADD45b expression mediates delayed activation of p38 MAP kinase by TGF-β. Embo J. 21, 6473–6482 (2002).
Hoffmeyer, A., Piekorz, R., Moriggl, R. & Ihle, J.N. Gadd45γ is dispensable for normal mouse development and T-cell proliferation. Mol. Cell. Biol. 21, 3137–43 (2001).
Zhang, W. et al. CR6: A third member in the MyD118 and Gadd45 gene family which functions in negative growth control. Oncogene 18, 4899–4907 (1999).
Lang, R., Patel, D., Morris, J.J., Rutschman, R.L. & Murray, P.J. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 169, 2253–2263 (2002).
Fan, W., Richter, G., Cereseto, A., Beadling, C. & Smith, K.A. Cytokine response gene 6 induces p21 and regulates both cell growth and arrest. Oncogene 18, 6573–6582 (1999).
Fornace, A.J. Jr., Jackman, J., Hollander, M.C., Hoffman-Liebermann, B. & Liebermann, D.A. Genotoxic-stress-response genes and growth-arrest genes. gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann. NY Acad. Sci. 663, 139–153 (1992).
Hollander, M.C. et al. Genomic instability in Gadd45α-deficient mice. Nat. Genet. 23, 176–184 (1999).
Kearsey, J.M., Coates, P.J., Prescott, A.R., Warbrick, E. & Hall, P.A. Gadd45 is a nuclear cell cycle regulated protein which interacts with p21Cip1. Oncogene 11, 1675–83 (1995).
Takekawa, M. & Saito, H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998).
Vairapandi, M., Balliet, A., Fornace, A.J. Jr., Hoffman, B. & Liebermann, D.A. The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene 12, 2579–2594 (1996).
Zhan, Q. et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18, 2892–2900 (1999).
Smith, M.L. et al. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 266, 1376–1380 (1994).
Harkin, D.P. et al. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97, 575–586 (1999).
Hildesheim, J. et al. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62, 7305–7315 (2002).
Takekawa, M., Posas, F. & Saito, H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16, 4973–4982 (1997).
Gerwins, P., Blank, J.L. & Johnson, G.L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 272, 8288–8295 (1997).
Azam, N., Vairapandi, M., Zhang, W., Hoffman, B. & Liebermann, D.A. Interaction of CR6 (GADD45γ) with proliferating cell nuclear antigen impedes negative growth control. J. Biol. Chem. 276, 2766–2774 (2001).
Zhao, H. et al. The central region of Gadd45 is required for its interaction with p21/WAF1. Exp. Cell Res. 258, 92–100 (2000).
Yi, Y.W. et al. Gadd45 family proteins are coactivators of nuclear hormone receptors. Biochem. Biophys. Res. Commun. 272, 193–198 (2000).
Kovalsky, O., Lung, F.D., Roller, P.P. & Fornace, A.J. Jr. Oligomerization of human Gadd45a protein. J. Biol. Chem. 276, 39330–39339 (2001).
Vairapandi, M., Azam, N., Balliet, A.G., Hoffman, B. & Liebermann, D.A. Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 and Gadd45-mediated negative growth control. J. Biol. Chem. 275, 16810–16819 (2000).
Weiss, L. et al. Regulation of c-Jun NH2-terminal kinase (Jnk) gene expression during T cell activation. J. Exp. Med. 191, 139–146 (2000).
Rincon, M. et al. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 17, 2817–2829 (1998).
Ladel, C.H., Flesch, I.E., Arnoldi, J. & Kaufmann, S.H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153, 3116–3122 (1994).
Hsieh, C.S. et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260, 547–549 (1993).
Shen, H. et al. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92, 3987–3991 (1995).
Pan, Z.K., Ikonomidis, G., Lazenby, A., Pardoll, D. & Paterson, Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat. Med. 1, 471–477 (1995).
Morinobu, A. et al. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc. Natl. Acad. Sci. USA 99, 12281–12286 (2002).
Huang, Q. et al. The plasticity of dendritic cell responses to pathogens and their components. Science 294, 870–875 (2001).
Jin, R. et al. Regulation of the gadd45β promoter by NF-κB. DNA Cell. Biol. 21, 491–503 (2002).
Wang, X., Gorospe, M. & Holbrook, N.J. Gadd45 is not required for activation of c-Jun N-terminal kinase or p38 during acute stress. J. Biol. Chem. 274, 29599–29602 (1999).
Shaulian, E. & Karin, M. Stress-induced JNK activation is independent of Gadd45 induction. J. Biol. Chem. 274, 29595–29598 (1999).
Dong, C., Davis, R.J. & Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Lu, B. et al. Genome wide analysis reveals key molecular circuitries that control T cell activation and Th1/Th2 differentiation. Proc. Natl. Acad. Sci. USA (in the press).
Kursar, M. et al. Organ-specific CD4+ T cell response during Listeria monocytogenes infection. J. Immunol. 168, 6382–6387 (2002).
Acknowledgements
We thank E. Eynon for scientific interactions; E. Tran for suggestions and reading the manuscript; L. Evangelisti, J. Miller and J. Stein for technical assistance; G. Chenell for secretarial assistance; and F. Manzo for assistance with manuscript preparation. We also thank Wyeth (formerly Genetics Institute) for the gift of recombinant IL-12. Supported by National Institutes of Health grants (1 P01 AI36529 and 1 K01 AR048854) and the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lu, B., Ferrandino, A. & Flavell, R. Gadd45β is important for perpetuating cognate and inflammatory signals in T cells. Nat Immunol 5, 38–44 (2004). https://doi.org/10.1038/ni1020
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1020
This article is cited by
-
ALV-J inhibits autophagy through the GADD45β/MEKK4/P38MAPK signaling pathway and mediates apoptosis following autophagy
Cell Death & Disease (2020)
-
Gadd45β promotes regeneration after injury through TGFβ-dependent restitution in experimental colitis
Experimental & Molecular Medicine (2019)
-
Effects of microgravity simulation on zebrafish transcriptomes and bone physiology—exposure starting at 5 days post fertilization
npj Microgravity (2016)
-
Harnessing downstream NF-κB signalling to achieve apoptosis-inducing anti-cancer-specific activity
Cell Death & Disease (2016)
-
Phosphorylation of Atg5 by the Gadd45β–MEKK4-p38 pathway inhibits autophagy
Cell Death & Differentiation (2013)