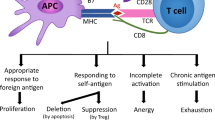
Abstract
Cellular immunity mediated by CD8+ T cells plays an indispensable role in bacterial and viral clearance and cancers. However, persistent antigen stimulation of CD8+ T cells leads to an exhausted or dysfunctional cellular state characterized by the loss of effector function and high expression of inhibitory receptors during chronic viral infection and in tumors. Numerous studies have shown that glycogen synthase kinase 3 (GSK3) controls the function and development of immune cells, but whether GSK3 affects CD8+ T cells is not clearly elucidated. Here, we demonstrate that mice with deletion of Gsk3α and Gsk3β in activated CD8+ T cells (DKO) exhibited decreased CTL differentiation and effector function during acute and chronic viral infection. In addition, DKO mice failed to control tumor growth due to the upregulated expression of inhibitory receptors and augmented T-cell exhaustion in tumor-infiltrating CD8+ T cells. Strikingly, anti-PD-1 immunotherapy substantially restored tumor rejection in DKO mice. Mechanistically, GSK3 regulates T-cell exhaustion by suppressing TCR-induced nuclear import of NFAT, thereby in turn dampening NFAT-mediated exhaustion-related gene expression, including TOX/TOX2 and PD-1. Thus, we uncovered the molecular mechanisms underlying GSK3 regulation of CTL differentiation and T-cell exhaustion in anti-tumor immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
References
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10.
Denton AE, et al. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8 + T cells. Proc Natl Acad Sci USA. 2011;108:15306–11.
Jenkins MR, et al. Cell cycle-related acquisition of cytotoxic mediators defines the progressive differentiation to effector status for virus-specific CD8 + T cells. J Immunol. 2008;181:3818–22.
Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9.
Doering TA, et al. Network analysis reveals centrally connected genes and pathways involved in CD8 + T cell exhaustion versus memory. Immunity. 2012;37:1130–44.
Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4.
Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61.
He R, et al. PD-1 and CTLA-4 inhibitors in combination vs. alone for the treatment of advanced melanoma: a systematic review and meta-analysis. Med (Baltim). 2022;101:e30561.
Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56.
Araki K, Youngblood B, Ahmed R. Programmed cell death 1-directed immunotherapy for enhancing T-cell function. Cold Spring Harb Symp Quant Biol. 2013;78:239–47.
Martinez GJ, et al. The transcription factor NFAT promotes exhaustion of activated CD8( + ) T cells. Immunity. 2015;42:265–78.
Man K, et al. Transcription factor IRF4 promotes CD8( + ) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity. 2017;47:1129–41 e5.
Chen Y, et al. BATF regulates progenitor to cytolytic effector CD8( + ) T cell transition during chronic viral infection. Nat Immunol. 2021;22:996–1007.
Seo H, et al. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat Immunol. 2021;22:983–95.
Tsui C, et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature. 2022;609:354–60.
Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10:645–56.
Seo H, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8( + ) T cell exhaustion. Proc Natl Acad Sci USA. 2019;116:12410–12415.
Khan O, et al. TOX transcriptionally and epigenetically programs CD8( + ) T cell exhaustion. Nature. 2019;571:211–18.
Scott AC, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–74.
Alfei F, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–69.
Yao C, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8( + ) T cell persistence in chronic infection. Nat Immunol. 2019;20:890–901.
Fiol CJ, et al. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–8.
Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–7.
ter Haar E, et al. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8:593–6.
Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–23.
Beals CR, et al. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–4.
Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010;31:24–31.
Ohteki T, et al. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J Exp Med. 2000;192:99–104.
Taylor A, et al. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8(+) cytolytic T cell responses. Immunity. 2016;44:274–86.
Rudd CE, Chanthong K, Taylor A. Small molecule inhibition of GSK-3 specifically inhibits the transcription of inhibitory co-receptor LAG-3 for enhanced anti-tumor immunity. Cell Rep. 2020;30:2075–82 e4.
Pearce EL, et al. Control of effector CD8 + T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3.
Intlekofer AM, et al. Effector and memory CD8 + T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44.
Liang C, et al. TOX as a potential target for immunotherapy in lymphocytic malignancies. Biomark Res. 2021;9:20.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Liu C, et al. Glycogen synthase kinase 3 drives thymocyte egress by suppressing beta-catenin activation of Akt. Sci Adv. 2021;7:eabg6262.
Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–79.
Kurachi M, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8 + T cells. Nat Immunol. 2014;15:373–83.
Chen L, et al. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42–8.
Duran J, et al. GSK-3beta/NFAT signaling is involved in testosterone-induced cardiac myocyte hypertrophy. PLoS One. 2016;11:e0168255.
Kim MS, et al. Nerve growth factor (NGF) regulates activity of nuclear factor of activated T-cells (NFAT) in neurons via the phosphatidylinositol 3-kinase (PI3K)-Akt-glycogen synthase kinase 3beta (GSK3beta) pathway. J Biol Chem. 2014;289:31349–60.
Acknowledgements
We thank all members of the Liu Labs (Xiamen University) for the discussion and technical assistance. We thank Xiaohong Ma and Xiufeng Sun at the Xiamen University Flow Cytometry Core and Suqin Wu in the XMU Laboratory Animal Center for technical assistance.
Funding
This study was supported by the National Natural Science Foundation of China (31770953, 81830047, and 81961138008 to CX and 32070877 to W-HL), 1000 Young Talents Program of China (NX), and the Fundamental Research Funds for the Central Universities of China-Xiamen University (20720170064 to CX). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YF conceived the study. YF, JW, and CL performed most of the mouse, cell, and molecular biology experiments. YF and JW performed RNA sequencing experiments. RT and KL analyzed the RNA sequencing data. YF, JW, and XG performed several of the molecular experiments. W-HL cosupervised the project. YF and W-HL wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Y., Wang, J., Liu, C. et al. Glycogen synthase kinase 3 controls T-cell exhaustion by regulating NFAT activation. Cell Mol Immunol 20, 1127–1139 (2023). https://doi.org/10.1038/s41423-023-01075-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-023-01075-0