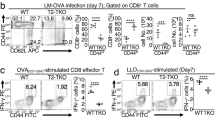
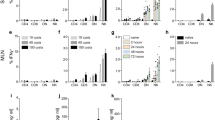
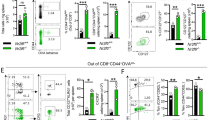
Abstract
Nod2 belongs to the nucleotide-binding oligomerization domain receptor (NLR) family of proteins, which function as intracellular pathogen sensors in innate immune cells. Nod2 deficiency results in an impaired immune response to bacterial pathogens. However, how this protein promotes host defense against intracellular parasites is unknown. Here we found that Nod2−/− mice had less clearance of Toxoplasma gondii and lower interferon-γ (IFN-γ) production. Reconstitution of T cell–deficient mice with Nod2−/− T cells followed by T. gondii infection demonstrated a T cell–intrinsic defect. Nod2−/− CD4+ T cells had poor helper T cell differentiation, which was associated with impaired production of interleukin 2 (IL-2) and nuclear accumulation of the transcription factor subunit c-Rel. Our data demonstrate a T cell–intrinsic role for Nod2 signaling that is critical for host defense against T. gondii.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Shaw, M.H., Reimer, T., Kim, Y.G. & Nuñez, G. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr. Opin. Immunol. 20, 377–382 (2008).
Chen, G., Shaw, M.H., Kim, Y.G. & Nuñez, G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol 4, 365–398 (2009).
Denkers, E.Y., Gazzinelli, R.T., Martin, D. & Sher, A. Emergence of NK1.1+ cells as effectors of IFN-γ dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J. Exp. Med. 178, 1465–1472 (1993).
Denkers, E.Y. & Gazzinelli, R.T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11, 569–588 (1998).
Yarovinsky, F. et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 (2005).
Mun, H.S. et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 15, 1081–1087 (2003).
Aliberti, J. et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4, 485–490 (2003).
Park, J.H. et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386 (2007).
Sher, A., Oswald, I.P., Hieny, S. & Gazzinelli, R.T. Toxoplasma gondii induces a T-independent IFN-γ response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-α. J. Immunol. 150, 3982–3989 (1993).
Shaw, M.H. et al. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-γ-dependent IL-10 reactivation. J. Immunol. 176, 7263–7271 (2006).
Reis e Sousa, C. et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186, 1819–1829 (1997).
Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2, 547–556 (2002).
Morrissey, P.J., Charrier, K., Braddy, S., Liggitt, D. & Watson, J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 178, 237–244 (1993).
Powrie, F., Leach, M.W., Mauze, S., Caddle, L.B. & Coffman, R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
Fraser, J.D., Irving, B.A., Crabtree, G.R. & Weiss, A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 251, 313–316 (1991).
Zhou, X.Y. et al. Molecular mechanisms underlying differential contribution of CD28 versus non-CD28 costimulatory molecules to IL-2 promoter activation. J. Immunol. 168, 3847–3854 (2002).
Kontgen, F. et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9, 1965–1977 (1995).
Sanchez-Valdepenas, C., Martin, A.G., Ramakrishnan, P., Wallach, D. & Fresno, M. NF-κB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. J. Immunol. 176, 4666–4674 (2006).
Yamada, T. et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-κB-inducing kinase. J. Immunol. 165, 804–812 (2000).
Pan, Q. et al. NF-κB-inducing kinase regulates selected gene expression in the Nod2 signaling pathway. Infect. Immun. 74, 2121–2127 (2006).
Gaide, O. et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3, 836–843 (2002).
Wang, D. et al. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat. Immunol. 3, 830–835 (2002).
Bradley, P.J. & Sibley, L.D. Rhoptries: an arsenal of secreted virulence factors. Curr. Opin. Microbiol. 10, 582–587 (2007).
Carruthers, V.B. & Sibley, L.D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73, 114–123 (1997).
Malek, T.R. & Bayer, A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 4, 665–674 (2004).
Bream, J.H. et al. A distal region in the interferon-γ gene is a site of epigenetic remodeling and transcriptional regulation by interleukin-2. J. Biol. Chem. 279, 41249–41257 (2004).
Liao, W. et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor α-chain expression. Nat. Immunol. 9, 1288–1296 (2008).
D'Souza, W.N., Schluns, K.S., Masopust, D. & Lefrancois, L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J. Immunol. 168, 5566–5572 (2002).
Liu, Z. et al. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J. Immunol. 167, 1830–1838 (2001).
Gudmundsdottir, H. & Turka, L.A. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J. Immunol. 167, 3699–3707 (2001).
Hagen, K.A. et al. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J. Immunol. 173, 3909–3915 (2004).
Yen, M.H., Lepak, N. & Swain, S.L. Induction of CD4 T cell changes in murine AIDS is dependent on costimulation and involves a dysregulation of homeostasis. J. Immunol. 169, 722–731 (2002).
Civil, A., Rensink, I., Aarden, L.A. & Verweij, C.L. Functional disparity of distinct CD28 response elements toward mitogenic responses. J. Biol. Chem. 274, 34369–34374 (1999).
Sun, S.C. & Ley, S.C. New insights into NF-κB regulation and function. Trends Immunol. 29, 469–478 (2008).
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001).
Hara, H. et al. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18, 763–775 (2003).
Kobe, B. & Kajava, A.V. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 (2001).
Hall, H.T. et al. RIP2 contributes to Nod signaling but is not essential for T cell proliferation, T helper differentiation or TLR responses. Eur. J. Immunol. 38, 64–72 (2008).
Nembrini, C., Reissmann, R., Kopf, M. & Marsland, B.J. Effective T-cell immune responses in the absence of the serine/threonine kinase RIP2. Microbes Infect. 10, 522–530 (2008).
Magalhaes, J.G. et al. Nod2-dependent Th2 polarization of antigen-specific immunity. J. Immunol. 181, 7925–7935 (2008).
Kobayashi, K.S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Moreira, L.O. et al. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine 26, 5808–5813 (2008).
Greenwald, R.J., Freeman, G.J. & Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 9, 271–285 (2009).
Villegas, E.N., Lieberman, L.A., Carding, S.R. & Hunter, C.A. Susceptibility of interleukin-2-deficient mice to Toxoplasma gondii is associated with a defect in the production of γ interferon. Infect. Immun. 70, 4757–4761 (2002).
Mason, N.J., Liou, H.C. & Hunter, C.A. T cell-intrinsic expression of c-Rel regulates Th1 cell responses essential for resistance to Toxoplasma gondii. J. Immunol. 172, 3704–3711 (2004).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Acknowledgements
We thank A. Weiss (University of California, San Francisco) for the pCD28RE/AP-1 luc reporter plasmid; G.Y. Chen for critically reading the manuscript; S. Koonse for assistance with managing mice colonies; the University of Michigan Flow Cytometry and the immunology core facilities for assistance with flow cytometry and enzyme-linked immunosorbent assay (ELISA); and G.S. Yap for discussions. Supported by the US National Institutes of Health (R01 DK61707; and T32-HL007517 to M.H.S.), the Swiss National Science Foundation (T.R.), the University of Michigan Comprehensive Cancer Center (Y.-G.K.), the Spanish Ministerio de Ciencia e Innovacion (SAF2007-61716 to M.F.), European Union (Eicosanox, for M.F.), Comunidad de Madrid (S-SAL-0159/2006 to M.F.) and La Red Temática de Investigación Cooperativa en Enfermedades Cardiovasculares (RD06/0014/1013 to M.F.).
Author information
Authors and Affiliations
Contributions
M.H.S. developed the concept and designed experiments; C.S.-V. and M.F. designed and did Jurkat and CD28RE–AP-1 assays; T.R., N.W. and Y.-G.K. provided technical support and conceptual advice; M.H.S. and G.N. prepared the manuscript; G.N. directed the research; and all authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1354 kb)
Rights and permissions
About this article
Cite this article
Shaw, M., Reimer, T., Sánchez-Valdepeñas, C. et al. T cell–intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol 10, 1267–1274 (2009). https://doi.org/10.1038/ni.1816
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1816
This article is cited by
-
Microbial ligand-independent regulation of lymphopoiesis by NOD1
Nature Immunology (2023)
-
Impact of parasitic infection on human gut ecology and immune regulations
Translational Medicine Communications (2021)
-
Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells
Parasites & Vectors (2021)
-
T cell-intrinsic role for Nod2 in protection against Th17-mediated uveitis
Nature Communications (2020)
-
Exploring the TLR and NLR signaling pathway relevant molecules induced by the Theileria annulata infection in calves
Parasitology Research (2018)