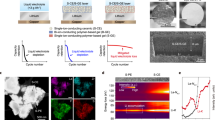
Abstract
The development of lithium–air batteries is plagued by a high potential gap (>1.2 V) between charge and discharge, and poor cyclability due to the drastic phase change of O2 (gas) and Ox− (condensed phase) at the cathode during battery operations. Here we report a cathode consisting of nanoscale amorphous lithia (nanolithia) confined in a cobalt oxide, enabling charge/discharge between solid Li2O/Li2O2/LiO2 without any gas evolution. The cathode has a theoretical capacity of 1,341 Ah kg−1, a mass density exceeding 2.2 g cm−3, and a practical discharge capacity of 587 Ah kg−1 at 2.55 V versus Li/Li+. It also displays stable cycling performance (only 1.8% loss after 130 cycles in lithium-matched full-cell tests against Li4Ti5O12 anode), as well as a round-trip overpotential of only 0.24 V. Interestingly, the cathode is automatically protected from O2 gas release and overcharging through the shuttling of self-generated radical species soluble in the carbonate electrolyte.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhu, Z. et al. Precise preparation of high performance spherical hierarchical LiNi0.5Mn1.5O4 for 5 V lithium ion secondary batteries. J. Mater. Chem. A 1, 5492–5496 (2013).
Zhu, Z. et al. Preparation of 4.7 V cathode material LiNi0.5Mn1.5O4 by an oxalic acid-pretreated solid-state method for lithium-ion secondary battery. J. Power Sources 224, 13–19 (2013).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Lu, J. et al. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 114, 5611–5640 (2014).
Débart, A., Paterson, A. J., Bao, J. & Bruce, P. G. α-MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. 120, 4597–4600 (2008).
Adams, B. D. et al. Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ. Sci. 6, 1772–1778 (2013).
Kushima, A. et al. Charging/discharging nanomorphology asymmetry and rate-dependent capacity degradation in Li–oxygen battery. Nano Lett. 15, 8260–8265 (2015).
Lu, Y.-C. et al. Platinum-gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium–air batteries. J. Am. Chem. Soc. 132, 12170–12171 (2010).
Zhai, D. Y. et al. Interfacial effects on lithium superoxide disproportionation in Li–O2 batteries. Nano Lett. 15, 1041–1046 (2015).
Steininger, H., Lehwald, S. & Ibach, H. Adsorption of oxygen on Pt (111). Surf. Sci. 123, 1–17 (1982).
Jones, R. D., Summerville, D. A. & Basolo, F. Synthetic oxygen carriers related to biological systems. Chem. Rev. 79, 139–179 (1979).
Qi, L., Qian, X. & Li, J. Near neutrality of an oxygen molecule adsorbed on a Pt (111) surface. Phys. Rev. Lett. 101, 146101 (2008).
Cabana, J., Monconduit, L., Larcher, D. & Palacin, M. R. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Kang, S., Mo, Y., Ong, S. P. & Ceder, G. A facile mechanism for recharging Li2O2 in Li–O2 batteries. Chem. Mater. 25, 3328–3336 (2013).
Laoire, C. et al. Rechargeable lithium/TEGDME-LiPF6/O2 battery. J. Electrochem. Soc. 158, A302–A308 (2011).
Laoire, C. O. et al. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium–air battery. J. Phys. Chem. C 114, 9178–9186 (2010).
Lu, Y.-C. et al. The influence of catalysts on discharge and charge voltages of rechargeable Li–oxygen batteries. Electrochem. Solid-State Lett. 13, A69–A72 (2010).
Wang, C. et al. Slurryless Li2S/reduced graphene oxide cathode paper for high-performance lithium sulfur battery. Nano Lett. 15, 1796–1802 (2015).
Okuoka, S.-i. et al. A new sealed lithium–peroxide battery with a co-doped Li2O cathode in a superconcentrated lithium Bis(fluorosulfonyl)amide electrolyte. Sci. Rep. 4, 5684 (2014).
Zhang, L., Zhang, Z.-C. & Amine, K. in Lithium Ion Batteries - New Developments (ed. Belharouak, I. ) (InTech, 2012).
Yang, Z. H. et al. Glass transition dynamics and surface layer mobility in unentangled polystyrene films. Science 328, 1676–1679 (2010).
Shin, K. et al. Enhanced mobility of confined polymers. Nature Mater. 6, 961–965 (2007).
Ellison, C. J. & Torkelson, J. M. The distribution of glass-transition temperatures in nanoscopically confined glass formers. Nature Mater. 2, 695–700 (2003).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li–O2 batteries. Nature Chem. 6, 1091–1099 (2014).
Zhai, D. et al. Raman evidence for late stage disproportionation in a Li–O2 Battery. J. Phys. Chem. Lett. 5, 2705–2710 (2014).
Zhang, M. et al. Size-dependent melting point depression of nanostructures: nanocalorimetric measurements. Phys. Rev. B 62, 10548–10557 (2000).
Lau, K. C., Curtiss, L. A. & Greeley, J. Density functional investigation of the thermodynamic stability of lithium oxide bulk crystalline structures as a function of oxygen pressure. J. Phys. Chem. C 115, 23625–23633 (2011).
Kao, Y.-H. et al. Overpotential-dependent phase transformation pathways in lithium iron phosphate battery electrodes. Chem. Mater. 22, 5845–5855 (2010).
Li, S. et al. High-rate aluminium yolk-shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity. Nature Commun. 6, 7872 (2015).
Bryantsev, V. S. & Blanco, M. Computational study of the mechanisms of superoxide-induced decomposition of organic carbonate-based electrolytes. J. Phys. Chem. Lett. 2, 379–383 (2011).
Freunberger, S. A. et al. Reactions in the rechargeable lithium–O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 133, 8040–8047 (2011).
Yang, Y. et al. New nanostructured Li2S/silicon rechargeable battery with high specific energy. Nano Lett. 10, 1486–1491 (2010).
Acknowledgements
We acknowledge financial support by NSF DMR-1410636. We thank Z. Wang for assistance with TEM measurements and analysis. We also thank H. Yao for help with the NMR data analysis and layout of the figures. This work was also partially supported by the US Department of Energy under Contract DE-AC0206CH11357 from the Vehicle Technologies Office, Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE).
Author information
Authors and Affiliations
Contributions
J.Li and Z.Z. conceived the original idea of this paper. Z.Z. performed the material synthesis and measurements, and then improved the experiments after discussions with J.Li and J.Lu. Z.Y. assisted with the Raman and XPS experiments; A.K. performed the TEM experiments and NMR calculation. Z.Z. and J.Li drafted the paper, and all authors revised it. L.Q. and K.A. provided many suggestions and guidance.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–9, Supplementary References (PDF 817 kb)
Rights and permissions
About this article
Cite this article
Zhu, Z., Kushima, A., Yin, Z. et al. Anion-redox nanolithia cathodes for Li-ion batteries. Nat Energy 1, 16111 (2016). https://doi.org/10.1038/nenergy.2016.111
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.111
This article is cited by
-
Developing in situ electron paramagnetic resonance characterization for understanding electron transfer of rechargeable batteries
Nano Research (2023)
-
Solid electrolyte interphase on anodes in rechargeable lithium batteries
Nano Research (2023)
-
Interfacial reactions in lithia-based cathodes depending on the binder in the electrode and salt in the electrolyte
Scientific Reports (2022)
-
Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage
Nature Reviews Materials (2022)
-
A high-energy-density and long-life initial-anode-free lithium battery enabled by a Li2O sacrificial agent
Nature Energy (2021)