Abstract
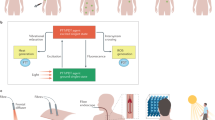
Debate is ongoing about the treatment of organ-confined prostate cancer, particularly in men who have low-risk disease detected by PSA screening. A balance is needed between the harms and benefits of treatment. New techniques are being developed that aim to offer similar treatment effects to current radical therapies, while reducing the associated harmful effects of these treatments. In this Review, we explore the potential of one such technique, photodynamic therapy (PDT), for the treatment of organ-confined prostate cancer. PDT uses a photosensitizing drug that is activated in the prostate by low-power laser light, delivered using optical fibers. The fibers are placed within needles in the prostate, guided by transrectal ultrasound and a perineal template. Following the activation of the photosensitizer by light, and the formation of reactive oxygen species, necrosis occurs at the site of interaction between the photosensitizer, light and oxygen. Clinical studies are underway to investigate the use of PDT for primary and salvage treatment of organ-confined prostate cancer. We review these studies, the potential strategies for enhanced photodynamic effects, and the current limitations of PDT for prostate cancer.
Key Points
-
Photodynamic therapy is under investigation for the treatment of organ-confined prostate cancer
-
Photodynamic therapy involves the activation of a photosensitizing drug, by light of a specific wavelength, in the presence of oxygen to produce reactive oxygen species, which are responsible for localized tissue destruction
-
Improvements in photosensitizer design and light delivery have resulted in consistently better outcomes
-
Much potential exists for further improvements in photosensitizer design, light delivery and treatment monitoring, which could improve the clinical outcomes in men with previously untreated prostate cancer, or those with recurrence after radiotherapy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Klotz L (2008) Active surveillance for prostate cancer: trials and tribulations. World J Urol 26: 437–442
Parker C et al. (2006) A model of the natural history of screen-detected prostate cancer, and the effect of radical treatment on overall survival. Br J Cancer 94: 1361–1368
Klotz L (2005) Active surveillance with selective delayed intervention is the way to manage 'good-risk' prostate cancer. Nat Clin Pract Urol 2: 136–142
Barqawi AB and Crawford ED (2007) The current use and future trends of focal surgical therapy in the management of localized prostate cancer. Cancer J 13: 313–317
Polascik TJ and Mouraviev V (2008) Focal therapy for prostate cancer. Curr Opin Urol 18: 269–274
Eggener SE et al. (2007) Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol 178: 2260–2267
Onik G (2004) The male lumpectomy: rationale for a cancer targeted approach for prostate cryoablation. A review. Technol Cancer Res Treat 3: 365–370
Poissonnier L et al. (2007) Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol 51: 381–387
Uchida T et al. (2006) Five years experience of transrectal high-intensity focused ultrasound using the Sonablate device in the treatment of localized prostate cancer. Int J Urol 13: 228–233
Shariat SF et al. (2005) Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate 65: 260–267
Korbelik M (2006) PDT-associated host response and its role in the therapy outcome. Lasers Surg Med 38: 500–508
Von Tappeiner H and Jodlbauer A (1907) The sensitizing action of fluorescent substances. An overall account of investigations on photodynamic phenomena [German]. Leipzig FCW Vogel
Lou PJ et al. (2004) Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br J Cancer 91: 441–446
Bown SG et al. (2002) Photodynamic therapy for cancer of the pancreas. Gut 50: 549–557
Gold MH (2007) Acne vulgaris: lasers, light sources and photodynamic therapy—an update 2007. Expert Rev Anti Infect Ther 5: 1059–1069
Wormald R et al. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No.: CD002030. 10.1002/14651858.CD002030.pub3
Huang Z et al. (2006) Magnetic resonance imaging correlated with the histopathological effect of Pd-bacteriopheophorbide (Tookad) photodynamic therapy on the normal canine prostate gland. Lasers Surg Med 38: 672–681
Trachtenberg J et al. (2008) Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int 102: 556–562
Trachtenberg J et al. (2007) Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. J Urol 178: 1974–1979
Haider MA et al. (2007) Prostate gland: MR imaging appearance after vascular targeted photodynamic therapy with palladium-bacteriopheophorbide. Radiology 244: 196–204
Gross S et al. (2003) Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat Med 9: 1327–1331
Fleshker S et al. (2008) Prompt assessment of WST11-VTP outcome using luciferase transfected tumors enables second treatment and increase in overall therapeutic rate. Photochem Photobiol 84: 1231–1237
Wink M et al. (2008) Contrast-enhanced ultrasound and prostate cancer: a multicentre European research coordination project. Eur Urol 54: 982–993
Pantelides ML et al. (1990) Photodynamic therapy for localised prostatic cancer: light penetration in the human prostate gland. J Urol 143: 398–401
Chen Q et al. (1993) Photodynamic therapy in prostate cancer: optical dosimetry and response of normal tissue. Proc SPIE 1881: 231–235
Whitehurst C et al. (1994) In vivo laser light distribution in human prostatic carcinoma. J Urol 151: 1411–1415
Lee LK et al. (1995) In situ comparison of 665 nm and 633 nm wavelength light penetration in the human prostate gland. Photochem Photobiol 62: 882–886
Lee LK et al. (1999) An interstitial light assembly for photodynamic therapy in prostatic carcinoma. BJU Int 84: 821–826
Zhu TC et al. (2005) Optical properties of human prostate at 732 nm measured in mediated photodynamic therapy. Photochem Photobiol 81: 96–105
Li J and Zhu TC (2008) Determination of in vivo light fluence distribution in a heterogeneous prostate during photodynamic therapy. Phys Med Biol 53: 2103–2114
Yu G et al. (2006) Real-time in situ monitoring of human prostate photodynamic therapy with diffuse light. Photochem Photobiol 82: 1279–1284
Zhu TC et al. (2005) Determination of the distribution of light, optical properties, drug concentration, and tissue oxygenation in vivo in human prostate during motexafin lutetium-mediated photodynamic therapy. J Photochem Photobiol B 79: 231–241
Finlay JC et al. (2006) Interstitial fluorescence spectroscopy in the human prostate during motexafin lutetium-mediated photodynamic therapy. Photochem Photobiol 82: 1270–1278
Moore C et al. (2006) A clinical evaluation of the optical characteristics of the prostate in men with prostate cancer [abstract #433]. Eur Urol 5 (Suppl): 131
Johansson A et al. (2007) Realtime light dosimetry software tools for interstitial photodynamic therapy of the human prostate. Med Phys 34: 4309–4321
Altschuler MD et al. (2005) Optimized interstitial PDT prostate treatment planning with the Cimmino feasibility algorithm. Med Phys 32: 3524–3536
Thompson MS et al. (2005) Clinical system for interstitial photodynamic therapy with combined on-line dosimetry measurements. Appl Opt 44: 4023–4031
Jankun J et al. (2005) Diverse optical characteristic of the prostate and light delivery system: implications for computer modelling of prostatic photodynamic therapy. BJU Int 95: 1237–1244
Windahl T et al. (1990) Photodynamic therapy of localised prostatic cancer. Lancet 336: 1139
Nathan TR et al. (2002) Photodynamic therapy for prostate cancer recurrence after radiotherapy: a phase I study. J Urol 168: 1427–1432
Chang SC et al. (1996) Interstitial and transurethral photodynamic therapy of the canine prostate using meso-tetra-(m-hydroxyphenyl) chlorin. Int J Cancer 67: 555–562
Chang SC et al. (1997) Interstitial photodynamic therapy in the canine prostate with disulfonated aluminum phthalocyanine and 5-aminolevulinic acid-induced protoporphyrin IX. Prostate 32: 89–98
Moore CM et al. (2006) Photodynamic therapy using meso tetra hydroxy phenyl chlorin (mTHPC) in early prostate cancer. Lasers Surg Med 38: 356–363
Zaak D et al. (2003) Photodynamic therapy by means of 5-ALA induced PPIX in human prostate cancer—preliminary results. Medical Laser Application 18: 91–95
Hsi RA et al. (2001) Photodynamic therapy in the canine prostate using motexafin lutetium. Clin Cancer Res 7: 651–660
Pinthus JH et al. (2006) Photodynamic therapy for urological malignancies: past to current approaches. J Urol 175: 1201–1207
Verigos K et al. (2006) Updated results of a phase I trial of motexafin lutetium-mediated interstitial photodynamic therapy in patients with locally recurrent prostate cancer. J Environ Pathol Toxicol Oncol 25: 373–387
Patel H et al. (2008) Motexafin lutetium-photodynamic therapy of prostate cancer: short- and long-term effects on prostate-specific antigen. Clin Cancer Res 14: 4869–4876
Zhu TC et al. (2003) In vivo optical properties of normal canine prostate at 732 nm using motexafin lutetium-mediated photodynamic therapy. Photochem Photobiol 77: 81–88
Weersink RA et al. (2005) Assessment of cutaneous photosensitivity of TOOKAD (WST09) in preclinical animal models and in patients. Photochem Photobiol 81: 106–113
Moore C et al. (2006) Vascular-targeted photodynamic therapy in organ confined prostate cancer–report of a novel photosensitiser [abstract #434]. Eur Urol 5 (Suppl): 131
Berdugo M et al. (2008) Evaluation of the new photosensitizer Stakel (WST-11) for photodynamic choroidal vessel occlusion in rabbit and rat eyes. Invest Ophthalmol Vis Sci 49: 1633–1644
Mazor O et al. (2005) WST11, a novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem Photobiol 81: 342–351
Curnow A et al. (2006) Comparing and combining light dose fractionation and iron chelation to enhance experimental photodynamic therapy with aminolevulinic acid. Lasers Surg Med 38: 325–331
Togashi H et al. (2006) Fractionated photodynamic therapy for a human oral squamous cell carcinoma xenograft. Oral Oncol 42: 526–532
Dolmans DE et al. (2002) Targeting tumor vasculature and cancer cells in orthotopic breast tumor by fractionated photosensitizer dosing photodynamic therapy. Cancer Res 62: 4289–4294
Bisland SK et al. (2004) Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors. Photochem Photobiol 80: 22–30
Renno RZ et al. (2004) Selective photodynamic therapy by targeted verteporfin delivery to experimental choroidal neovascularization mediated by a homing peptide to vascular endothelial growth factor receptor-s2. Arch Ophthalmol 122: 1002–1011
Demidova TN and Hamblin MR (2004) Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol 17: 245–254
Embleton ML et al. (2005) Development of a novel targeting system for lethal photosensitization of antibiotic-resistant strains of Staphylococcus aureus . Antimicrob Agents Chemother 49: 3690–3696
Embleton ML et al. (2004) Antibody-directed photodynamic therapy of methicillin-resistant Staphylococcus aureus . Microb Drug Resist 10: 92–97
Savellano MD et al. (2005) Multiepitope HER2 targeting enhances photoimmunotherapy of HER2-overexpressing cancer cells with pyropheophorbide-a immunoconjugates. Cancer Res 65: 6371–6379
Vrouenraets MB et al. (2002) Comparison of aluminium (III) phthalocyanine tetrasulfonate- and meta-tetrahydroxyphenylchlorin-monoclonal antibody conjugates for their efficacy in photodynamic therapy in vitro . Int J Cancer 98: 793–798
Sharman WM et al. (2004) Targeted photodynamic therapy via receptor mediated delivery systems. Adv Drug Deliv Rev 56: 53–76
Schneider R et al. (2005) Design, synthesis, and biological evaluation of folic acid targeted tetraphenylporphyrin as novel photosensitizers for selective photodynamic therapy. Bioorg Med Chem 13: 2799–2808
Ichikawa K et al. (2004) PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy. Biol Pharm Bull 27: 443–444
Hamblin MR et al. (2003) Pegylation of charged polymer-photosensitiser conjugates: effects on photodynamic efficacy. Br J Cancer 89: 937–943
van Nostrum CF (2004) Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Deliv Rev 56: 9–16
Chen X et al. (2004) Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: targeting and incapacitating cancer cells. Biochemistry 43: 10918–10929
Hamblin M et al. (2000) Scavenger-receptor targeted photodynamic therapy. Photochem Photobiol 72: 533–540
Acknowledgements
Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Caroline M Moore, Doug Pendse and Mark Emberton have declared that they are each involved in ongoing clinical studies to evaluate the use of palladium bacteropheophorbide vascular-targeted photodynamic therapy (WST-09, WST-11) as a first-line treatment in men with organ-confined prostate cancer. They have all acted as Consultants for Steba Biotech and have received honoraria for this work. The clinical studies are supported by Steba Biotech.
Rights and permissions
About this article
Cite this article
Moore, C., Pendse, D. & Emberton, M. Photodynamic therapy for prostate cancer—a review of current status and future promise. Nat Rev Urol 6, 18–30 (2009). https://doi.org/10.1038/ncpuro1274
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro1274
This article is cited by
-
High photoluminescence and afterglow emission of nitrogen-doped graphene quantum dots/TiO2 nanocomposite for use as a photodynamic therapy photosensitizer
Applied Physics A (2024)
-
Enhancing photodynamic inactivation via tunning spatial constraint on photosensitizer
Science China Chemistry (2024)
-
Carbon quantum dots/Bi4O5Br2 photocatalyst with enhanced photodynamic therapy: killing of lung cancer (A549) cells in vitro
Rare Metals (2022)
-
A novel hierarchical targeting and controllable smart nanoparticles for enhanced in situ nuclear photodynamic therapy
Nano Research (2022)
-
Tumour irradiation combined with vascular-targeted photodynamic therapy enhances antitumour effects in pre-clinical prostate cancer
British Journal of Cancer (2021)