Abstract
IgA nephropathy is the most common form of primary glomerulonephritis. Variations in clinical manifestations indicate that a diagnosis of IgA nephropathy encompasses multiple disease subsets that cannot be distinguished on the basis of renal pathology or clinical variables alone. Familial forms of the disease have been reported throughout the world, but are probably under-recognized because associated urinary abnormalities are often intermittent in affected family members. IgA nephropathy has complex determination, with different genes probably causing disease in different patient subgroups. Of the many pathogenic mechanisms reported, defects in IgA1 glycosylation that lead to formation of immune complexes have been consistently implicated. Here, we present the evidence for genetic contributions to the disease, review clinical patterns of familial disease, and summarize some of the most promising genetic studies conducted to date. Linkage-based approaches to the study of familial forms of the disease have identified significant or suggestive loci on chromosomes 6q22-23, 2q36, 4q26-31, 17q12-22 and 3p24-23, but no causal gene has yet been identified. Many interesting, but poorly replicated, genetic association studies have also been reported. We discuss recent developments in analytic tools that should enable genetic studies of sporadic forms of disease by the genome-wide association approach.
Key Points
-
Up to 15% of cases of the common primary glomerulonephritis IgA nephropathy (IgAN) are classified as 'familial', indicating an underlying genetic component
-
Familial IgAN is probably underdiagnosed, as urinary abnormalities that cause clinical suspicion can occur intermittently
-
Analysis of extended kindreds has shown that some patients with 'sporadic' forms of IgAN share common ancestors
-
Families affected by IgAN are often affected by other glomerular diseases such as IgM nephropathy, Henoch-Schönlein purpura nephritis and focal segmental glomerulosclerosis
-
Autosomal dominant inheritance with incomplete penetrance is a likely form of transmission in families with IgAN
-
Linkage-based genetic studies have detected associations between IgAN and several chromosomal loci, but no specific disease-causing gene has been identified
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
van Es LA et al. (1988) Composition of IgA-containing circulating immune complexes in IgA nephropathy. Am J Kidney Dis 12: 397–401
Baldree LA et al. (1993) Immunoglobulin A-fibronectin aggregate levels in children and adults with immunoglobulin A nephropathy. Am J Kidney Dis 22: 1–4
Stratta P et al. (1996) Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis 27: 631–639
Suzuki K et al. (2003) Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294
Cosyns JP et al. (1998) Lesions in donor kidneys: nature, incidence, and influence on graft function. Transpl Int 11: 22–27
Rosenberg HG et al. (1990) Morphological findings in 70 kidneys of living donors for renal transplant. Pathol Res Pract 186: 619–624
Waldherr R et al. (1989) Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant 4: 943–946
D'Amico G (1987) The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727
Levy M and Berger J (1988) Worldwide perspective of IgA nephropathy. Am J Kidney Dis 12: 340–347
Glassock RJ et al. (1985) IgA nephropathy in Japan. Am J Nephrol 5: 127–137
Power DA et al. (1985) IgA nephropathy is not a rare disease in the United Kingdom. Nephron 40: 180–184
Taguchi T et al. (1987) A comparative study of IgA nephritis in Japan and Germany: an approach to its geopathology. Pathol Res Pract 182: 358–367
Abu-Romeh SH et al. (1989) Renal diseases in Kuwait: experience with 244 renal biopsies. Int Urol Nephrol 21: 25–29
Cheong I et al. (1991) IgA nephropathy in Malaysia. Southeast Asian J Trop Med Public Health 22: 120–122
Briganti EM et al. (2001) The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant 16: 1364–1367
Yamagata K et al. (2002) Prognosis of asymptomatic hematuria and/or proteinuria in men: high prevalence of IgA nephropathy among proteinuric patients found in mass screening. Nephron 91: 34–42
Gesualdo L et al. (2004) The Italian experience of the national registry of renal biopsies. Kidney Int 66: 890–894
Research Group on Progressive Chronic Renal Disease (1999) Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Nephron 82: 205–213
Galla JH et al. (1984) Racial difference in the prevalence of IgA-associated nephropathies. Lancet 2: 522
Smith SM and Hoy WE (1989) Frequent association of mesangial glomerulonephritis and alcohol abuse: a study of 3 ethnic groups. Mod Pathol 2: 138–143
Smith SM and Tung KS (1985) Incidence of IgA-related nephritides in American Indians in New Mexico. Hum Pathol 16: 181–184
Hoy WE et al. (1993) Mesangial proliferative glomerulonephritis in southwestern American Indians. Am J Kidney Dis 21: 486–496
Hoy WE and Megill DM (1989) End-stage renal disease in southwestern Native Americans, with special focus on the Zuni and Navajo Indians. Transplant Proc 21: 3906–3908
Hughson MD et al. (1989) Mesangiopathic glomerulonephritis in Zuni (New Mexico) Indians. Arch Pathol Lab Med 113: 148–157
O'Connell PJ et al. (1987) Familial IgA nephropathy: a study of renal disease in an Australian aboriginal family. Aust N Z J Med 17: 27–33
Casiro OG et al. (1988) The prevalence of IgA nephropathy in Manitoba Native Indian children. Can J Public Health 79: 308–310
Wyatt RJ et al. (1998) Epidemiology of IgA nephropathy in central and eastern Kentucky for the period 1975 through 1994. Central Kentucky Region of the Southeastern United States IgA Nephropathy DATABANK Project. J Am Soc Nephrol 9: 853–858
Sehic AM et al. (1997) Increased recognition of IgA nephropathy in African-American children. Pediatr Nephrol 11: 435–437
Barratt J and Feehally J (2005) IgA nephropathy. J Am Soc Nephrol 16: 2088–2097
Galla JH (1995) IgA nephropathy. Kidney Int 47: 377–387
Donadio JV and Grande JP (2002) IgA nephropathy. N Engl J Med 347: 738–748
Tomana M et al. (1997) Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int 52: 509–516
Tomana M et al. (1999) Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81
Kerr MA (1990) The structure and function of human IgA. Biochem J 271: 285–296
Mattu TS et al. (1998) The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions. J Biol Chem 273: 2260–2272
Iwasaki H et al. (2003) Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-alpha-L-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem 278: 5613–5621
Field MC et al. (1989) O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans 17: 1034–1035
Baenziger JU and Maynard Y (1980) Human hepatic lectin: physiochemical properties and specificity. J Biol Chem 255: 4607–4613
Tomana M et al. (1985) Carbohydrate-mediated clearance of secretory IgA from the circulation. Mol Immunol 22: 887–892
Allen AC et al. (1995) Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol 100: 470–474
Allen AC et al. (2001) Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int 60: 969–973
Hiki Y et al. (2001) Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int 59: 1077–1085
Smith AC et al. (2006) O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol 17: 1192–1199
Roccatello D et al. (1993) Removal systems of immunoglobulin A and immunoglobulin A containing complexes in IgA nephropathy and cirrhosis patients: the role of asialoglycoprotein receptors. Lab Invest 69: 714–723
Novak J et al. (2005) IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504–513
Moura IC et al. (2004) Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15: 622–634
Moura IC et al. (2005) Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. J Am Soc Nephrol 16: 2667–2676
Greer MR et al. (1998) The nucleotide sequence of the IgA1 hinge region in IgA nephropathy. Nephrol Dial Transplant 13: 1980–1983
Allen AC et al. (1997) Leucocyte beta 1,3 galactosyltransferase activity in IgA nephropathy. Nephrol Dial Transplant 12: 701–706
Allen AC et al. (1998) Abnormal IgA glycosylation in Henoch-Schönlein purpura restricted to patients with clinical nephritis. Nephrol Dial Transplant 13: 930–934
Ju T and Cummings RD (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci U S A 99: 16613–16618
Ju T et al. (2002) Cloning and expression of human core 1 beta1,3-galactosyltransferase. J Biol Chem 277: 178–186
Ju T and Cummings RD (2005) Protein glycosylation: chaperone mutation in Tn syndrome. Nature 437: 1252
Kudo T et al. (2002) Molecular cloning and characterization of a novel UDP-Gal:GalNAc(alpha) peptide beta 1,3-galactosyltransferase (C1Gal-T2), an enzyme synthesizing a core 1 structure of O-glycan. J Biol Chem 277: 47724–47731
Moldoveanu Z et al. (2007) Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int [doi:10.1038/sj.ki.5002185]
Gharavi A et al. (2006) Are glycosylation defects heritable in familial IgA nephropathy (IgAN) [abstract]? J Am Soc Nephrol 17: 253A
Koushik R et al. (2005) Persistent, asymptomatic, microscopic hematuria in prospective kidney donors. Transplantation 80: 1425–1429
Vadivel N et al. (2007) Accepting prospective kidney donors with asymptomatic urinary abnormalities: are we shooting in the dark? Kidney Int 71: 173–177
Karnib HH et al. (2007) Characterization of large Lebanese family segregating IgA nephropathy. Nephrol Dial Transplant 22: 772–777
Tolkoff-Rubin NE et al. (1978) IgA nephropathy in HLA-identical siblings. Transplantation 26: 430–433
Sabatier JC et al. (1979) Mesangial IgA glomerulonephritis in HLA-identical brothers. Clin Nephrol 11: 35–38
Masuda J et al. (1996) Identical twin sisters with IgA nephropathy [Japanese]. Nippon Jinzo Gakkai Shi 38: 52–56
Rambausek M et al. (1985) Hypertension in mesangial IgA glomerulonephritis. Proc Eur Dial Transplant Assoc Eur Ren Assoc 21: 693–697
Kabasakal C et al. (1997) IgA nephropathy occurring in two siblings of three families. Turk J Pediatr 39: 395–401
Johnston PA et al. (1992) Clinico-pathological correlations and long-term follow-up of 253 United Kingdom patients with IgA nephropathy: a report from the MRC Glomerulonephritis Registry. Q J Med 84: 619–627
Rambausek M et al. (1987) Familial glomerulonephritis. Pediatr Nephrol 1: 416–418
Schena FP et al. (1993) IgA nephropathy: pros and cons for a familial disease. Contrib Nephrol 104: 36–45
Scolari F et al. (1999) Familial clustering of IgA nephropathy: further evidence in an Italian population. Am J Kidney Dis 33: 857–865
Levy M (1989) Familial cases of Berger's disease and anaphylactoid purpura: more frequent than previously thought. Am J Med 87: 246–248
Levy M (1989) Familial cases of Berger's disease or of Berger's disease and rheumatoid purpura. Cooperative study of the Societe Francaise de Nephrologie [French]. Nephrologie 10: 175–182
Julian BA et al. (1985) Familial IgA nephropathy: evidence of an inherited mechanism of disease. N Engl J Med 312: 202–208
Wyatt RJ et al. (1987) Regionalization in hereditary IgA nephropathy. Am J Hum Genet 41: 36–50
Izzi C et al. (2006) Familial aggregation of primary glomerulonephritis in an Italian population isolate: Valtrompia study. Kidney Int 69: 1033–1040
Levy M (2001) Familial cases of Berger's disease and anaphylactoid purpura. Kidney Int 60: 1611–1612
Schena FP (1995) Immunogenetic aspects of primary IgA nephropathy. Kidney Int 48: 1998–2013
Scolari F et al. (1992) Familial occurrence of primary glomerulonephritis: evidence for a role of genetic factors. Nephrol Dial Transplant 7: 587–596
Levy M (1993) Multiplex families in IgA nephropathy. Contrib Nephrol 104: 46–53
Schena FP et al. (2002) Increased risk of end-stage renal disease in familial IgA nephropathy. J Am Soc Nephrol 13: 453–460
Izzi C et al. (2006) IgA nephropathy: the presence of familial disease does not confer an increased risk for progression. Am J Kidney Dis 47: 761–769
Frasca GM et al. (2004) Thin basement membrane disease in patients with familial IgA nephropathy. J Nephrol 17: 778–785
Scolari F (1999) Familial IgA nephropathy. J Nephrol 12: 213–219
Frasca GM et al. (2000) Thin glomerular basement membrane disease. J Nephrol 13: 15–19
Julian BA et al. (1991) Macroscopic hematuria and proteinuria preceding renal IgA deposition in patients with IgA nephropathy. Am J Kidney Dis 17: 472–479
Lasseur C et al. (1997) Henoch-Schönlein purpura with immunoglobulin A nephropathy and abnormalities of immunoglobulin A in a Wiskott-Aldrich syndrome carrier. Am J Kidney Dis 29: 285–287
Miyagawa S et al. (1989) Anaphylactoid purpura and familial IgA nephropathy. Am J Med 86: 340–342
Coppo R et al. (1999) Clinical features of Henoch-Schönlein purpura. Italian Group of Renal Immunopathology. Ann Med Interne (Paris) 150: 143–150
Fervenza FC (2003) Henoch-Schönlein purpura nephritis. Int J Dermatol 42: 170–177
Patel U et al. (1988) Henoch-Schönlein purpura after influenza vaccination. Br Med J (Clin Res Ed) 296: 1800
Sola Alberich R et al. (1997) Henoch-Schönlein purpura associated with acetylsalicylic acid. Ann Intern Med 126: 665
Nielsen HE (1988) Epidemiology of Schonlein-Henoch purpura. Acta Paediatr Scand 77: 125–131
Yang YH et al. (2005) A nationwide survey on epidemiological characteristics of childhood Henoch-Schönlein purpura in Taiwan. Rheumatology (Oxford) 44: 618–622
Cakir N et al. (2004) Henoch-Schönlein purpura in two brothers imprisoned in the same jail: presentation two months apart. Clin Exp Rheumatol 22: 235–237
Cosio FG et al. (1994) Association of thin glomerular basement membrane with other glomerulopathies. Kidney Int 46: 471–474
Berthoux FC et al. (1995) Primary IgA glomerulonephritis with thin glomerular basement membrane: a peculiar pathological marker versus thin membrane nephropathy association. Contrib Nephrol 111: 1–6
Yoshida K et al. (1998) A case of IgA nephropathy in three sisters with thin basement membrane disease. Am J Nephrol 18: 422–424
Savige J et al. (2003) Thin basement membrane nephropathy. Kidney Int 64: 1169–1178
Frasca GM et al. (2002) Two different glomerular diseases in the same patient at an interval of 7 years. Nephrol Dial Transplant 17: 2014–2016
Linossier MT et al. (2003) Different glycosylation profile of serum IgA1 in IgA nephropathy according to the glomerular basement membrane thickness: normal versus thin. Am J Kidney Dis 41: 558–564
Risch N and Merikangas K (1996) The future of genetic studies of complex human diseases. Science 273: 1516–1517
Lander ES and Botstein D (1986) Mapping complex genetic traits in humans: new methods using a complete RFLP linkage map. Cold Spring Harb Symp Quant Biol 51: 49–62
Lander ES and Schork NJ (1994) Genetic dissection of complex traits. Science 265: 2037–2048
Gharavi AG et al. (2000) IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet 26: 354–357
Bisceglia L et al. (2006) Genetic heterogeneity in Italian families with IgA nephropathy: suggestive linkage for two novel IgA nephropathy loci. Am J Hum Genet 79: 1130–1134
Beerman I et al. (2004) Replication of linkage and refinement of IGAN1 locus [abstract]. Am J Hum Genet 75A: 1897P
Pei Y et al. (2005) Localization of a novel disease susceptibility locus to chromosome 2q36 by genome scan of a large extended Canadian family with IgA nephropathy (IgAN) [abstract]. J Am Soc Nephrol 16 (Suppl): 592A
Kruglyak L (1999) Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet 22: 139–144
Heutink P and Oostra BA (2002) Gene finding in genetically isolated populations. Hum Mol Genet 11: 2507–2515
Peltonen L (2000) Positional cloning of disease genes: advantages of genetic isolates. Hum Hered 50: 66–75
Ott J and Hoh J (2000) Statistical approaches to gene mapping. Am J Hum Genet 67: 289–294
Reich DE and Lander ES (2001) On the allelic spectrum of human disease. Trends Genet 17: 502–510
Risch NJ (2000) Searching for genetic determinants in the new millennium. Nature 405: 847–856
Corder EH et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921–923
Takei T et al. (2002) Association between single-nucleotide polymorphisms in selectin genes and immunoglobulin A nephropathy. Am J Hum Genet 70: 781–786
Akiyama F et al. (2002) Single-nucleotide polymorphisms in the class II region of the major histocompatibility complex in Japanese patients with immunoglobulin A nephropathy. J Hum Genet 47: 532–538
Thibaudin L et al. (2004) G protein beta3 subunit C825T polymorphism in primary IgA nephropathy. Kidney Int 66: 322–328
Ohtsubo S et al. (2005) Association of a single-nucleotide polymorphism in the immunoglobulin mu-binding protein 2 gene with immunoglobulin A nephropathy. J Hum Genet 50: 30–35
Tuglular S et al. (2003) Polymorphisms of the tumour necrosis factor alpha gene at position -308 and TNFd microsatellite in primary IgA nephropathy. Nephrol Dial Transplant 18: 724–731
Masutani K et al. (2003) Impact of interferon-gamma and interleukin-4 gene polymorphisms on development and progression of IgA nephropathy in Japanese patients. Am J Kidney Dis 41: 371–379
Carturan S et al. (2004) Association between transforming growth factor beta1 gene polymorphisms and IgA nephropathy. J Nephrol 17: 786–793
Li YJ et al. (2004) Family-based association study showing that immunoglobulin A nephropathy is associated with the polymorphisms 2093C and 2180T in the 3′ untranslated region of the Megsin gene. J Am Soc Nephrol 15: 1739–1743
Li GS et al. (2007) Variants of C1GALT1 gene are associated with the genetic susceptibility to IgA nephropathy. Kidney Int 71: 448–453
Kim YS et al. (2001) Uteroglobin gene polymorphisms affect the progression of immunoglobulin A nephropathy by modulating the level of uteroglobin expression. Pharmacogenetics 11: 299–305
Narita I et al. (2002) Role of uteroglobin G38A polymorphism in the progression of IgA nephropathy in Japanese patients. Kidney Int 61: 1853–1858
Matsunaga A et al. (2002) Association of the uteroglobin gene polymorphism with IgA nephropathy. Am J Kidney Dis 39: 36–41
Harden PN et al. (1995) Polymorphisms in angiotensin-converting-enzyme gene and progression of IgA nephropathy. Lancet 345: 1540–1542
Pei Y et al. (1997) Association of angiotensinogen gene T235 variant with progression of immunoglobin A nephropathy in Caucasian patients. J Clin Invest 100: 814–820
Yoshioka T et al. (1998) Deletion polymorphism of the angiotensin converting enzyme gene predicts persistent proteinuria in Henoch-Schönlein purpura nephritis. Arch Dis Child 79: 394–399
Yoshida H et al. (1995) Role of the deletion of polymorphism of the angiotensin converting enzyme gene in the progression and therapeutic responsiveness of IgA nephropathy. J Clin Invest 96: 2162–2169
Berthoux FC et al. (2006) CC-chemokine receptor five gene polymorphism in primary IgA nephropathy: the 32 bp deletion allele is associated with late progression to end-stage renal failure with dialysis. Kidney Int 69: 565–572
Panzer U et al. (2005) The chemokine receptor 5 Delta32 mutation is associated with increased renal survival in patients with IgA nephropathy. Kidney Int 67: 75–81
Frimat L et al. (2000) Polymorphism of angiotensin converting enzyme, angiotensinogen, and angiotensin II type 1 receptor genes and end-stage renal failure in IgA nephropathy: IGARAS—a study of 274 men. J Am Soc Nephrol 11: 2062–2067
Santos NM et al. (2002) Angiotensin-converting enzyme genotype and outcome in pediatric IgA nephropathy. Pediatr Nephrol 17: 496–502
Altshuler D et al. (2005) A haplotype map of the human genome. Nature 437: 1299–1320
Grant SF et al. (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38: 320–323
Hirschhorn JN and Daly MJ (2005) Genome-wide association studies for common diseases and complex traits. Nat Rev Genet 6: 95–108
Klein RJ et al. (2005) Complement factor H polymorphism in age-related macular degeneration. Science 308: 385–389
Obara W et al. (2003) Association of single-nucleotide polymorphisms in the polymeric immunoglobulin receptor gene with immunoglobulin A nephropathy (IgAN) in Japanese patients. J Hum Genet 48: 293–299
Pe'er I et al. (2006) Evaluating and improving power in whole-genome association studies using fixed marker sets. Nat Genet 38: 663–66755
Acknowledgements
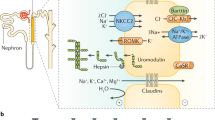
We thank the patients and their family members for participating in our research studies. This work is supported by NIH grants DK61525, DK71802, DK78244 and DK64400, and by the General Clinical Research Centers of the University of Alabama at Birmingham M01 RR00032, and the University of Tennessee Health Sciences Center M01 RR00211. AG Gharavi is supported by the Emerald Foundation and the National Kidney Foundation Clinical Scientist Program. We thank www.carbonwood.com for assistance with preparation of figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Beerman, I., Novak, J., Wyatt, R. et al. The genetics of IgA nephropathy. Nat Rev Nephrol 3, 325–338 (2007). https://doi.org/10.1038/ncpneph0492
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0492