Abstract
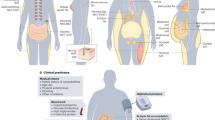
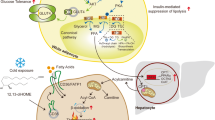
Abdominal fat accumulation has been shown to play crucial roles in the development of metabolic syndrome. Visceral fat accumulation particularly is closely correlated to the development of cardiovascular disease and obesity-related disorders such as diabetes mellitus, hyperlipidemia and hypertension. Given these clinical findings, the functions of adipocytes have been intensively investigated in the past 10 years, and have been revealed to act as endocrine cells that secrete various bioactive substances termed adipocytokines. Among adipocytokines, tumor-necrosis factor-α, plasminogen activator inhibitor type 1 and heparin-binding epidermal growth factor-like growth factor are produced in adipocytes as well as other organs, and contribute to the development of vascular diseases. Visfatin has been identified as a visceral-fat-specific protein that might be involved in the development of obesity-related diseases, such as diabetes mellitus and cardiovascular disease. In contrast to these adipocytokines, adiponectin, which is an adipose-tissue-specific, collagen-like protein, has been noted as an important antiatherogenic and antidiabetic protein, or as an anti-inflammatory protein. The functions of adipocytokine secretion might be regulated dynamically by nutritional state. Visceral fat accumulation causes dysregulation of adipocyte functions, including oversecretion of tumor-necrosis factor-α, plasminogen activator inhibitor type 1 and heparin-binding epidermal growth factor-like growth factor, and hyposecretion of adiponectin, which results in the development of a variety of metabolic and circulatory diseases. In this review, the importance of adipocytokines, particularly adiponectin, is discussed with respect to cardiovascular diseases.
Key Points
-
Intra-abdominal visceral fat accumulation has been recognized as having a major role in the development of cardiovascular disease
-
Adipocytes secrete various bioactive substances, termed adipocytokines, that contribute to metabolic dysregulation, abdominal fat accumulation and the development of vascular disease
-
Approximately 20% of all genes in subcutaneous adipose tissue and about 30% in visceral adipose tissue encode adipocytokine secretion
-
Plasma concentrations of adiponectin, a collagen-like protein, are reduced in individuals with visceral fat accumulation and type 2 diabetes, suggesting protective metabolic effects with high concentrations
-
The reduction of visceral fat, possibly with regulation of adipocytokines, might be a useful preventive measure for metabolic syndrome and related cardiovascular disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Reaven GM (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37: 1595–1607
Kaplan NM (1989) The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med 149: 1514–1520
DeFronzo RA (1991) Insulin resistance syndrome. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14: 173–194
International Diabetes Federation (online 14 April 2005) A new worldwide definition of the metabolic syndrome [http://www.idf.org/home/index.cfm?unode=32EF2063-B966-468F-928C-A5682A4E3910] (accessed 13 September 2005)
Matsuzawa Y (1997) Pathophysiology and molecular mechanism of visceral fat syndrome: The Japanese case. Diabetes Metab Rev 13: 3–13
Despres JP et al. (1989) Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes 38: 304–309
Maeda K et al. (1997) Analysis of expression profile of genes in the human adipose tissue. Gene 190: 227–235
Matsuzawa Y et al. (2004) Adiponectin and metabolic syndrome. Arteroscler Thromb Vasc Biol 24: 29–33
Maeda K et al. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221: 286–289
Vague J (1956) The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout and uric calculous disease. Am J Clin Nutr 4: 20–34
Fujioka S et al. (1987) Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 36: 54–59
Hulthe J et al. (2003) Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism 52: 1612–1614
Evans DJ et al. (1984) Relationship between skeletal muscle insulin resistance, insulin-mediated glucose disposal and insulin binding: Effect of obesity and body fat topography. J Clin Invest 74: 1515–1525
Yamashita S et al. (1996) Insulin resistance and body fat distribution. Diabetes Care 19: 287–291
Kanai H et al. (1990) Close correlation of intra-abdominal fat accumulation to hypertension in obese women. Hypertension 16: 484–490
Larson B et al. (1984) Abdominal adipose tissue distribution, obesity and risk of cardiovascular disease and death: 13 year follow up of participants in study of men born in 1913. BMJ 288: 1401–1410
Nakamura T et al. (1994) Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis 107: 239–246
Nakamura T et al. (1997) Importance of intraabdominal fat accumulation to coronary atherosclerosis in heterozygous familial hypercholesterolaemia. Int J Obes Relat Metab Disord 21: 580–586
Nakajima T et al. (1989) Correlation of intraabdominal fat accumulation and left ventricular performance in obesity. Am J Cardiol 64: 369–373
Shinohara E et al. (1997) Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Intern Med 241: 11–18
Funahashi T et al. (1999) Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Inter Med 38: 202–206
Uysal KT et al. (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389: 610–614
Shimomura I et al. (1996) Enhanced expression of PAI-1 in visceral fat: Possible contribution to vascular disease in obesity. Nat Med 2: 800–803
Matsumoto S et al. (2002) Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun 292: 781–786
Matsuzawa Y (2005) Adipocytokines and metabolic syndrome. Semin Vasc Med 5: 34–39
Cigolini M et al. (1996) Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 16: 368–374
Fukuhara A et al. (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307: 426–430
Hu E et al. (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703
Sherer EP et al. (1995) A novel serum protein similar to C1q produced exclusively in adipocytes. J Biol Chem 270: 26746–26749
Arita Y et al. (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83
Halleux CM et al. (2001) Secretion and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun 288: 1102–1107
Maeda N et al. (2001) PPARγ ligands increase expression and plasma concentration of adiponectin, an adipose-derived protein. Diabetes 50: 2094–2099
Hotta K et al. (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599
Stefan N et al. (2002) Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation and low plasma concentration precedes a decrease in whole body insulin sensitivity in humans. Diabetes 51: 1884–1888
Lindsay RS et al. (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360: 57–58
Maeda N et al. (2002) Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 8: 731–737
Tomas E et al. (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 90: 16309–16313
Iwashima Y et al. (2004) Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 43: 1318–1323
Ouchi N et al. (2003) Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42: 231–234
Ouchi N et al. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476
Zoccali C et al. (2002) Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol 13: 134–141
Kumada M et al. (2003) Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89
Pischon T et al. (2004) Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291: 1730–1737
Kondo H et al. (2002) Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 51: 2325–2328
Okamoto Y et al. (2002) Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 26: 2767–2770
Matsuda M et al. (2002) Role of adiponectin in preventing vascular stenosis: the missing link of adipo-vascular axis. J Biol Chem 277: 37487–37491
Okamoto Y et al. (2000) An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res 32: 47–50
Ouchi N et al. (2000) Adiponectin, adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through c-AMP dependent pathway. Circulation 102: 1296–1301
Arita Y et al. (2002) Adipocyte-derived plasma protein, adiponectin, acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation 105: 2893–2898
Ouchi N et al. (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103: 1057–1063
Kumada M et al. (2004) Adiponectin specifically increases tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109: 2046–2049
Shibata R et al. (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10: 1384–1389
Kobayashi H et al. (2004) Selective suppression of endothelial cell apotosis by high molecular weight form of adiponectin. Circ Res 94: e27–e31
Fisher FF et al. (2005) Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian Males. Diabetologia 48: 1084–1087
Hug C et al. (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101: 10308–10313
Yamauchi T et al. (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769
Nishizawa H et al. (2002) Small heterodimer partner, an orphan nuclear receptor, augments peroxisome proliferator-activated receptor γ transactivation. J Biol Chem 277: 1586–1592
Acknowledgements
Most of the research discussed in this review, including the clinical and cell-biology studies on adiponectin and visfatin, was done by the Adiposcience Group of Osaka University, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Matsuzawa, Y. Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Rev Cardiol 3, 35–42 (2006). https://doi.org/10.1038/ncpcardio0380
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0380
This article is cited by
-
Impacts of a 12-week aerobic, resistance, and combined exercise training on serum FAM19A5, glucose homeostasis, and novel cardiovascular risk factors among adults with obesity
International Journal of Diabetes in Developing Countries (2024)
-
High polygenic risk score for exceptional longevity is associated with a healthy metabolic profile
GeroScience (2023)
-
Adiponectin can be a good predictor of urodynamic detrusor underactivity: a prospective study in men with lower urinary tract symptoms
World Journal of Urology (2023)
-
An association between visceral or subcutaneous fat accumulation and diabetes mellitus among Japanese subjects
Diabetology & Metabolic Syndrome (2021)
-
Efficacy of verum and sham acupoint catgut embedding for treatment of obesity: Study protocol for a randomized controlled trial
Trials (2019)