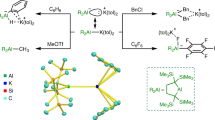
Abstract
The preparation of polyfunctional organometallics is important in organic synthesis as these reagents are very popular nucleophiles. The preparation of functionalized aluminium reagents by direct insertion of aluminium powder is in general not possible. Such a reaction would be of special importance owing to the low price of aluminium compared with magnesium (it is half the price), the low toxicity of this metal and the chemoselectivity of the resultant organoaluminium reagents. We have now found that by adding catalytic amounts of selected metallic chlorides (TiCl4, BiCl3, InCl3 or PbCl2) in the presence of LiCl, aluminium powder inserts into various unsaturated iodides and bromides under mild conditions. These resulting new organoaluminium reagents undergo smooth Pd-catalysed cross-coupling and acylation reactions, as well as copper-catalysed allylic substitutions, affording various interesting products for pharmaceutical and material science applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Knochel, P. Handbook of Functionalized Organometallics (Wiley-VCH, 2005).
Grignard, V. Mixed organomagnesium combinations and their application in acid, alcohol, and hydrocarbon synthesis. Ann. Chim. 24, 433–490 (1901).
Mole, T. & Jeffery, E. A. Organoaluminum Compounds (Elsevier, 1972).
Rieke, R. D. Preparation of organometallic compounds from highly reactive metal powders. Science 246, 1260–1264 (1989).
Rieke, R. D. & Hanson, M. V. New organometallic reagents using highly reactive metals. Tetrahedron 53, 1925–1956 (1997).
Lee, J., Velarde-Ortiz, R., Guijarro, A., Wurst, J. R. & Rieke, R. D. Low-temperature formation of functionalized Grignard reagents from direct oxidative addition of active magnesium to aryl bromides. J. Org. Chem. 65, 5428–5430 (2000).
Tilstam, U. & Weinmann, H. Activation of Mg metal for safe formation of Grignard reagents on plant scale. Org. Process Res. Dev. 6, 906–910 (2002).
Baker, K. V., Brown, J. M., Hughes, N., Skarnulis, A. J. & Sexton A. Mechanical activation of magnesium turnings for the preparation of reactive Grignard reagents. J. Org. Chem. 56, 698–703 (1991).
Knochel, P., Millot, N., Rodriguez, A. L. & Tucker, C. E. in Organic Reactions (ed. Overman, L. E.) Vol. 58 (Wiley-VCH, 2001).
Saito, S. in Comprehensive Organometallic Chemistry III (ed. Knochel, P.) Vol. 9 (Elsevier, 2007).
Uchiyama, M., Naka, H., Matsumoto, Y. & Ohwada, T. Regio- and chemoselective direct generation of functionalized aromatic aluminum compounds using aluminum ate base. J. Am. Chem. Soc. 126, 10526–10527 (2007).
Naka, H. et al. An aluminum ate base: Its design, structure, function, and reaction mechanism. J. Am. Chem. Soc. 129, 1921–1930 (2007).
Naka, H. et al. Mixed alkylamido aluminate as a kinetically controlled base. J. Am. Chem. Soc. 130, 16193–16200 (2008).
Wunderlich, S. & Knochel, P. Aluminum bases for the highly chemoselective preparation of aryl and heteroaryl aluminum compounds. Angew. Chem. Int. Ed. 48, 1501–1504 (2009).
Ishikawa, T., Ogawa, A. & Hirao, T. A novel oxovanadium(v)-induced oxidation of organoaluminum compounds. Highly selective coupling of organic substituents on aluminum. J. Am. Chem. Soc. 120, 5124–5125 (1998).
Hawner, C., Li, K., Cirriez, V. & Alexakis, A. Copper-catalyzed asymmetric conjugate addition of aryl aluminum reagents to trisubstituted enones: Construction of aryl-substituted quaternary centers. Angew. Chem. Int. Ed. 47, 8211–8214 (2008).
Westermann, J., Imbery, U., Nguyen, A. T. & Nickisch, K. Nickel-catalysed 1,4-addition of aryl groups to enones using arylalkylaluminum compounds. Eur. J. Inorg. Chem. 295–298 (1998).
Gao, H. & Knochel, P. New preparation and reactions of arylaluminum reagents using barbier conditions. Synlett 1321–1325 (2009).
Hallwachs, W. & Schafarik, A. Ueber die Verbindungen der Erdmetalle mit organischen Radicalen. Liebigs Ann. Chem. 109, 206–209 (1859).
Spencer, J. F. & Wallace, M. L. The interaction of metals of the aluminium group and organic halogen derivatives. J. Am. Chem. Soc. 93, 1827–1833 (1908).
Grosse, A. V. & Mavity, J. M. Organoaluminum compounds I. Methods of preparation. J. Org. Chem. 5, 106–121 (1940).
Krasovskiy, A., Malakhov, V., Gavryushin, A. & Knochel, P. Efficient synthesis of functionalized organozinc compounds by the direct insertion of zinc into organic iodides and bromides. Angew. Chem. Int. Ed. 45, 6040–6044 (2006).
Boudet, N., Sase S., Sinha, P., Liu, C.-Y., Krasovskiy, A. & Knochel, P. Directed ortho insertion (DoI): a new approach to functionalized aryl and heteroaryl zinc reagents. J. Am. Chem. Soc. 129, 12358–12359 (2007).
Metzger, A., Schade, M. A. & Knochel, P. LiCl-mediated preparation of highly functionalized benzylic zinc chlorides. Org. Lett. 10, 1107–1110 (2008).
Chen, Y.-H. & Knochel, P. Preparation of aryl and heteroaryl indium(iii) reagents by the direct insertion of indium in the presence of LiCl. Angew. Chem. Int. Ed. 47, 7648–7651 (2008).
Chen, Y.-H., Sun, M. & Knochel, P. LiCl-mediated preparation of functionalized benzylic indium(iii) halides and highly chemoselective palladium-catalyzed cross-coupling in a protic cosolvent. Angew. Chem. Int. Ed. 48, 2236–2239 (2009).
Piller, F. M., Appukkutan, P., Gavryushin, A., Helm, M. & Knochel, P. Convenient preparation of polyfunctional aryl magnesium reagents by a direct magnesium insertion in the presence of LiCl. Angew. Chem. Int. Ed. 47, 6802–6806 (2008).
Metzger, A., Piller, F. M. & Knochel, P. Polyfunctional benzylic zinc chlorides by the direct insertion of magnesium into benzylic chlorides in the presence of LiCl and ZnCl2 . Chem. Commun. 5824–5826 (2008).
Negishi, E.-i., Takahashi, T., Baba, S., Van Horn, D. E. & Okukado, N. Palladium- or nickel-catalyzed reactions of alkenylmetals with unsaturated organic halides as a selective route to arylated alkenes and conjugated dienes: Scope, limitations and mechanism. J. Am. Chem. Soc. 109, 2393–2401 (1987).
Negishi, E.-i. Palladium- or nickel-catalyzed cross coupling. A new selective method for carbon-carbon bond formation. Acc. Chem. Res. 15, 340–348 (1982).
Ku, S.-L., Hui, X.-P., Chen, C.-A., Kuo, Y.-Y. & Gau, H.-M. AlAr3(THF): Highly efficient reagents for cross-couplings with aryl bromides and chlorides catalyzed by the economic palladium complex of PCy3 . Chem. Commun. 3847–3849 (2007).
Zweifel, G. & Miller, J. A. in Organic Reactions (ed. Dauben W. G.) Vol. 32 (John Wiley & Sons, 1984).
Araki, S., Jin, S.-J., Idou, Y. & Butsugan, Y. Allylation of carbonyl compounds with catalytic amount of indium. Bull. Chem. Soc. Jpn 65, 1736–1738 (1992).
Takai, K. & Ikawa, Y. Indium-catalyzed reduction of allyl bromide with gallium or aluminum formation of allylgallium and allylaluminum sesquibromides. Org. Lett. 4, 1727–1729 (2002).
Augé, J., Lubin-Germain, N. & Thiaw-Woaye, A. Indium-catalyzed allylation of carbonyl compounds with the Mn/TMSCl system. Tetrahedron Lett. 20, 9245–9247 (1999).
Tanaka, H. & Kuroboshi, M. Aluminum as an electron pool for organic synthesis - multi-metal redox promoted reactions. Curr. Org. Chem. 8, 1027–1056 (2004).
Takai, K., Ueda, T., Hayashi, T. & Moriwake, T. Activation of manganese metal by a catalytic amount of PbCl2 and Me3SiCl. Tetrahedron Lett. 37, 7049–7052 (1996).
Takai, K., Ueda, T., Ikeda, N., Ishiyama, T. & Matsushita, H. Successive carbon-carbon bond formation by sequential generation of radical anionic species with manganese and catalytic amounts of PbCl2 and Me3SiCl. Bull. Chem. Soc. Jpn 76, 347–353 (2003).
Takai, K., Kakiuchi, T. & Utimoto, K. A dramatic effect of a catalytic amount of lead on the Simmons-Smith reaction and formation of alkylzinc compounds from iodoalkanes. Reactivity of zinc metal: Activation and deactivation. J. Org. Chem. 59, 2671–2673 (1994).
Zhang, X.-L., Han, Y., Tao, W.-T. & Huang, Y.-Z. PbCI2/Ga bimetal redox system-mediated carbon–carbon bond formation reactions between carbonyl compounds and ethyl trichloroacetate and lodoacetonitrile. J. Chem. Soc. Perkin Trans. 1 189–191 (1995).
Loh, T.-P. & Chua, G.-L. Discovery of indium complexes as water-tolerant Lewis acids. Chem. Commun. 2739–2749 (2006).
Araki, S. & Hirashita, T. in Comprehensive Organometallic Chemistry III (ed. Knochel, P.) (Pergamon Press, 2007).
Augé, J., Lubin-Germain, N. & Uziel, J. Recent advances in indium-promoted organic reactions. Synthesis 1739–1764 (2007).
O Brien, C. J. et al. Easily prepared air- and moisture-stable Pd-NHC (NHC=N-heterocyclic carbene) complexes: A reliable, user-friendly, highly active palladium precatalyst for the Suzuki-Miyaura reaction. Chem. Eur. J. 12, 4743–4748 (2006).
Organ, M. G., Calimsiz, S., Sayah, M., Hoi, K. H. & Lough, A. J. Pd-PEPPSI-IPent: An active, sterically demanding cross-coupling catalyst and its application in the synthesis of tetra-ortho-substituted biaryls. Angew. Chem. Int. Ed. 48, 2383–2387 (2009).
Srogl, J. Allred, G. D. & Liebeskind, L. S. Sulfonium salts. Participants par excellence in metal-catalyzed carbon-carbon bond-forming reactions. J. Am. Chem. Soc. 119, 12376–12377 (1997).
Prokopcov, H. & Kappe C. O. The Liebeskind-Srogl C–C cross-coupling reaction. Angew. Chem. Int. Ed. 48, 2276–2286 (2009).
Fausett, B. W. & Liebeskind, L. S. Palladium-catalyzed coupling of thiol esters with aryl and primary and secondary alkyl organoindium reagents. J. Org. Chem. 70, 4851–4853 (2005).
Villiéras, J. & Rambaud, M. Wittig-Horner reaction in heterogeneous media; 1. An easy synthesis of ethyl-hydroxymethylacrylate and ethyl-halomethylacrylates using formaldehyde in water. Synthesis 11, 924–926 (1982).
Knochel, P., Yeh, M. C. P., Berk, S. C. & Talbert, J. Synthesis and reactivity toward acyl chlorides and enones of the new highly functionalized copper reagents RCu(CN)ZnI. J. Org. Chem. 53, 2390–2392 (1988).
Acknowledgements
Y.-H.C. thanks the Humboldt Foundation for financial support. We thank the Fonds der Chemischen Industrie, the DFG and the European Research Council for financial support. We thank Chemetall and BASF for the generous gift of chemicals.
Author information
Authors and Affiliations
Contributions
T.B. and Y.-H.C. contributed equally to this work. Z.P. assisted in conducting and analysing the chemical experiments. P.K. designed and directed the project and wrote the manuscript with contributions from Y.-H.C. and T.B. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1342 kb)
Rights and permissions
About this article
Cite this article
Blümke, T., Chen, YH., Peng, Z. et al. Preparation of functionalized organoaluminiums by direct insertion of aluminium to unsaturated halides. Nature Chem 2, 313–318 (2010). https://doi.org/10.1038/nchem.590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.590
This article is cited by
-
Catalytic asymmetric carbon–carbon bond formation using alkenes as alkylmetal equivalents
Nature Chemistry (2012)