Abstract
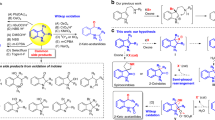
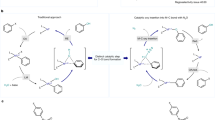
The development of sustainable oxidation chemistry demands strategies to harness O2 as a terminal oxidant. Oxidase catalysis, in which O2 serves as a chemical oxidant without necessitating incorporation of oxygen into reaction products, would allow diverse substrate functionalization chemistry to be coupled to O2 reduction. Direct O2 utilization suffers from intrinsic challenges imposed by the triplet ground state of O2 and the disparate electron inventories of four-electron O2 reduction and two-electron substrate oxidation. Here, we generate hypervalent iodine reagents—a broadly useful class of selective two-electron oxidants—from O2. This is achieved by intercepting reactive intermediates of aldehyde autoxidation to aerobically generate hypervalent iodine reagents for a broad array of substrate oxidation reactions. The use of aryl iodides as mediators of aerobic oxidation underpins an oxidase catalysis platform that couples substrate oxidation directly to O2 reduction. We anticipate that aerobically generated hypervalent iodine reagents will expand the scope of aerobic oxidation chemistry in chemical synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cavani, F. & Teles, J. H. Sustainability in catalytic oxidation: an alternative approach or a structural evolution? ChemSusChem 2, 508–534 (2009).
Campbell, A. N. & Stahl, S. S. Overcoming the ‘oxidant problem’: strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu). Acc. Chem. Res. 45, 851–863 (2012).
Wertz, S. & Studer, A. Nitroxide-catalyzed transition-metal-free aerobic oxidation processes. Green Chem. 15, 3116–3134 (2013).
Filatov, M., Reckien, W., Peyerimhoff, S. D. & Shaik, S. What are the reasons for the kinetic stability of a mixture of H2 and O2? J. Phys. Chem. A 104, 12014–12020 (2000).
Borden, W. T., Hoffmann, R., Stuyver, T. & Chen, B. Dioxygen: what makes this triplet diradical kinetically persistent? J. Am. Chem. Soc. 139, 9010–9018 (2017).
Ho, R. Y. N., Liebman, J. F. & Valentine, J. S. in Active Oxygen in Chemistry 1–23 (Blackie Academic and Professional, 1995).
McCann, S. D. & Stahl, S. S. Copper-catalyzed aerobic oxidations of organic molecules: pathways for two-electron oxidation with a four-electron oxidant and a one-electron redox-active catalyst. Acc. Chem. Res. 48, 1756–1766 (2015).
Stahl, S. S. Palladium oxidase catalysis: selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 43, 3400–3420 (2004).
Wendlandt, A. E. & Stahl, S. S. Quinone-catalyzed selective oxidation of organic molecules. Angew. Chem. Int. Ed. 54, 14638–14658 (2015).
Piera, J. & Bäckvall, J.-E. Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—a biomimetic approach. Angew. Chem. Int. Ed. 47, 3506–3523 (2008).
Yoshimura, A. & Zhdankin, V. V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 116, 3328–3435 (2016).
Zhdankin, V. V. Hypervalent Iodine Chemistry: Preparation, Structure and Synthetic Applications of Polyvalent Iodine Compounds (Wiley, 2014).
Varvoglis, A. Hypervalent Iodine in Organic Synthesis (Academic, 1997).
Brand, J. P., González, D. F., Nicolai, S. & Waser, J. Benziodoxole-based hypervalent iodine reagents for atom-transfer reactions. Chem. Commun. 47, 102–115 (2011).
Charpentier, J., Früh, N. & Togni, A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 115, 650–682 (2015).
Banik, S. M., Mennie, K. M. & Jacobsen, E. N. Catalytic 1,3-difunctionalization via oxidative C–C bond activation. J. Am. Chem. Soc. 139, 9152–9155 (2017).
Mu, R. et al. An efficient catalytic aerobic oxidation of alcohols in water using hypervalent iodine(V). Adv. Synth. Catal. 347, 1333–1336 (2005).
Uyanik, M., Fukatsu, R. & Ishihara, K. Bromine-catalyzed aerobic oxidation of alcohols. Chem. Asian J. 5, 456–460 (2010).
Reich, L. & Stivala, S. S. in Autoxidation of Hydrocarbons and Polyolefins 1–30 (Marcel Dekker, 1969).
Wöhler, F. & Liebig, J. Untersuchungen über das Radikal der Benzoesäure [In German]. Liebigs Ann. 3, 249–282 (1832).
Bäckström, H. L. J. The chain-reaction theory of negative catalysis. J. Am. Chem. Soc. 49, 1460–1472 (1927).
Baeyer, A. & Villiger, V. Benzoylwasserstoffsuperoxyd und die Oxydation des Benzaldehyds an der Luft [In German]. Ber. Dtsch Chem. Ges. 33, 1569–1585 (1900).
Chudasama, V., Fitzmaurice, R. J. & Caddick, S. Hydroacylation of α,β-unsaturated esters via aerobic C–H activation. Nat. Chem. 2, 592–596 (2010).
Chudasama, V., Fitzmaurice, R. J., Ahern, J. M. & Caddick, S. Dioxygen mediated hydroacylation of vinyl sulfonates and sulfones on water. Chem. Commun. 46, 133–135 (2010).
Chudasama, V., Ahern, J. M., Fitzmaurice, R. J. & Caddick, S. Synthesis of γ-ketophosphonates via aerobic hydroacylation of vinyl phosphonates. Tetrahedron Lett. 52, 1067–1069 (2011).
Chudasama, V., Akhbar, A. R., Bahou, K. A., Fitzmaurice, R. J. & Caddick, S. Metal-free hydroacylation of C=C and N=N bonds via aerobic C–H activation of aldehydes, and reaction of the products thereof. Org. Biomol. Chem. 11, 7301–7317 (2013).
Paul, S. & Guin, J. Dioxygen-mediated decarbonylative C–H alkylation of heteroaromatic bases with aldehydes. Chem. Eur. J. 21, 17618–17622 (2015).
Yamada, T., Takai, T., Rhode, O. & Mukaiyama, T. Direct epoxidation of olefins catalyzed by nickel(II) complexes with molecular oxygen and aldehydes. Bull. Chem. Soc. Jpn 64, 2109–2117 (1991).
Kaneda, K. et al. A convenient synthesis of epoxides from olefins using molecular oxygen in the absence of metal catalysts. Tetrahedron Lett. 33, 6827–6830 (1992).
Mizuno, N., Weiner, H. & Finke, R. G. Co-oxidative epoxidation of cyclohexene with molecular oxygen, isobutyraldehyde reductant, and the polyoxoanion-supported catalyst precursor [(n-C4H9)4N]5Na3[(1,5-COD)Ir·P2W15Nb3O62]. The importance of key control experiments including omitting the catalyst and adding radical-chain initiators. J. Mol. Catal. A 114, 15–28 (1996).
Nam, W., Kim, H. J., Kim, S. H., Ho, R. Y. N. & Valentine, J. S. Metal complex-catalyzed epoxidation of olefins by dioxygen with co-oxidation of aldehydes. A mechanistic study. Inorg. Chem. 35, 1045–1049 (1996).
Das, P., Saha, D., Saha, D. & Guin, J. Aerobic direct C(sp2)–H hydroxylation of 2-arylpyridines by palladium catalysis induced with aldehyde auto-oxidation. ACS Catal. 6, 6050–6054 (2016).
Larkin, D. R. The role of catalysts in the air oxidation of aliphatic aldehydes. J. Org. Chem. 55, 1563–1568 (1990).
Lehtinen, C. & Brunow, G. Factors affecting the selectivity of air oxidation of 2-ethyhexanal, an α-branched aliphatic aldehyde. Org. Process Res. Dev. 4, 544–549 (2000).
Müller, P. & Godoy, J. Ru-catalyzed oxidations with iodosylbenzene derivatives. Substituent effects on selectivity in oxidation of sulfides and alcohols. Helv. Chim. Acta 66, 1790–1795 (1983).
Katritzky, A. R., Gallos, J. K. & Durst, H. D. Structure of and electronic interactions in aromatic polyvalent iodine compounds: a 13C NMR study. Magn. Reson. Chem. 27, 815–822 (1989).
Koser, G. F., Wettach, R. H., Troup, J. M. & Frenz, B. A. Hypervalent iodine. Crystal structure of phenylhydroxytosyloxyiodine. J. Org. Chem. 41, 3609–3611 (1976).
Wolf, W., Chalekson, E. & Kobata, D. Structure and proof of structure of benzodiiodoxole. J. Org. Chem. 32, 3239–3241 (1967).
Macikenas, D., Skrzypczak-Jankun, E. & Protasiewicz, J. D. A new class of iodonium ylides engineered as soluble primary oxo and nitrene sources. J. Am. Chem. Soc. 121, 7164–7165 (1999). .
Zhong, W., Yang, J., Meng, X. & Li, Z. BF3·OEt2-promoted diastereoselective diacetoxylation of alkenes by PhI(OAc)2 . J. Org. Chem. 76, 9997–10004 (2011).
Yamamoto, Y. & Togo, H. PhI-catalyzed α-tosylation of ketones with m-chloroperbenzoic acid and p-toluenesulfonic acid. Synlett. 798–800 (2006).
Dohi, T. et al. Designer μ-oxo-bridged hypervalent iodine(III) organocatalysts for greener oxidations. Chem. Commun. 46, 7697–7699 (2010).
Lucchetti, N., Scalone, M., Fantasia, S. & Muñiz, K. An improved catalyst for iodine(I/III)-catalyzed intermolecular C–H amination. Adv. Synth. Catal. 358, 2093–2099 (2016).
Yablonskii, O. P., Vinogradov, M. G., Kereselidze, R. V. & Nikishin, G. I. Mechanism of the oxidation of aldehydes by oxygen. Bull. Acad. Sci. USSR 18, 272–275 (1969).
Miyamoto, K. et al. Iodoarene-catalyzed oxidative transformations using molecular oxygen. Chem. Commun. 53, 9781–9784 (2017).
Acknowledgements
The authors thank Texas A&M University and the Welch Foundation (A-1907) for financial support.
Author information
Authors and Affiliations
Contributions
A.M. and D.C.P. conceived of the project. A.M. and S.-M.H. carried out the experimental work. A.M., S.-M.H. and D.C.P. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A provisional patent has been filed on the aerobic oxidation of aryl iodides.
Supplementary information
Supplementary information
Supplementary information (PDF 2096 kb)
Rights and permissions
About this article
Cite this article
Maity, A., Hyun, SM. & Powers, D. Oxidase catalysis via aerobically generated hypervalent iodine intermediates. Nature Chem 10, 200–204 (2018). https://doi.org/10.1038/nchem.2873
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2873
This article is cited by
-
Achiral organoiodine-functionalized helical polyisocyanides for multiple asymmetric dearomative oxidations
Nature Communications (2023)
-
Oxidative Iododeborylation Reaction of (Hetero)arylboronic Acids in Water Extract of Pomegranate Ash: A Novel and Sustainable Synthesis of Iodo(hetero)arenes
Waste and Biomass Valorization (2022)