Abstract
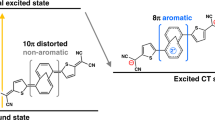
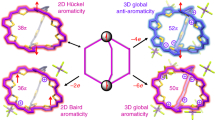
The reversal of (anti)aromaticity in a molecule's triplet excited state compared with its closed-shell singlet ground state is known as Baird's rule and has attracted the interest of synthetic, physical organic chemists and theorists because of the potential to modulate the fundamental properties of highly conjugated molecules. Here we show that two closely related bis-rhodium hexaphyrins (R26H and R28H) containing [26] and [28] π-electron peripheries, respectively, exhibit properties consistent with Baird's rule. In the ground state, R26H exhibits a sharp Soret-like band and distinct Q-like bands characteristic of an aromatic porphyrinoid, whereas R28H exhibits a broad absorption spectrum without Q-like bands, which is typical of an antiaromatic porphyrinoid. In contrast, the T–T absorption of R26H is broad, weak and featureless, whereas that of R28H displays an intense and sharp Soret-like band. These spectral signatures, in combination with quantum chemical calculations, are in line with qualitative expectations based on Baird's rule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Minkin, V. I., Glukhovtsev, M. N. & Simkin, B. Y. Aromaticity and Antiaromaticity: Electronic and Structural Aspects (Wiley, 1994).
Cyrański, M. K. Energetic aspects of cyclic π-electron delocalization: evaluation of the methods of estimating aromatic stabilization energies. Chem. Rev. 105, 3773–3811 (2005).
Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. Z. Phys. 70, 204–286 (1931).
Rzepa, H. S. Möbius aromaticity and delocalization. Chem. Rev. 105, 3697–3715 (2005).
Baird, N. C. Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3ππ* state of cyclic hydrocarbons. J. Am. Chem. Soc. 94, 4941–4948 (1972).
Ottosson, H. Organic photochemistry: exciting excited-state aromaticity. Nature Chem. 4, 969–971 (2012).
Rosenberg, M., Dahlstrand, C., Kilså, K. & Ottosson, H. Excited state aromaticity and antiaromaticity: opportunities for photophysical and photochemical rationalizations. Chem. Rev. 114, 5379–5425 (2014).
Aihara, J.-i. Aromaticity-based theory of pericyclic reactions. Bull. Chem. Soc. Jpn 51, 1788–1792 (1978).
Ilić, P., Sinković, B. & Trinajstić, N. Topological resonance energies of conjugated structures. Isr. J. Chem. 20, 258–269 (1980).
Fratev, F., Monev, V. & Janoschek, R. Ab initio study of cyclobutadiene in excited states: optimized geometries, electronic transitions and aromaticities. Tetrahedron 38, 2929–2932 (1982).
Gogonea, V., Schleyer, P. v. R. & Schreiner, P. R. Consequences of triplet aromaticity in 4nπ-electron annulenes: calculation of magnetic shieldings for open-shell species. Angew. Chem. Int. Ed. 37, 1945–1948 (1998).
Zilberg, S. & Haas, Y. Two-state model of antiaromaticity: the low lying singlet states. J. Phys. Chem. A 102, 10843–10850 (1998).
Krygowski, T. M. & Cyrański, M. K. Two sources of the decrease of aromaticity: bond length alternation and bond elongation. Part II. An analysis based on geometry of the singlet and triplet states of 4nπ annulenes: C4H4, C5H5+, C6H62+, C7H7−, C8H8, C9H9+. Tetrahedron 55, 11143–11148 (1999).
Fowler, P. W., Steiner, E. & Jennesken, L. W. Ring-current aromaticity in triplet states of 4nπ electron monocycles. Chem. Phys. Lett. 371, 719–723 (2003).
Villaume, S., Fogarty, H. A. & Ottosson, H. Triplet-state aromaticity of 4nπ-electron monocycles: analysis of bifurcation in the π contribution to the electron localization function. ChemPhysChem 9, 257–264 (2008).
Karadakov, P. B. Ground- and excited-state aromaticity and antiaromaticity in benzene and cyclobutadiene. J. Phys. Chem. A 112, 7303–7309 (2008).
Karadakov, P. B. Aromaticity and antiaromaticity in the low-lying electronic states of cyclooctatetraene. J. Phys. Chem. A 112, 12707–12713 (2008).
Feixas, F., Vandenbussche, J., Bultinck, P., Matito, E. & Solà, M. Electron delocalization and aromaticity in low-lying excited states of archetypal organic compounds. Phys. Chem. Chem. Phys. 13, 20690–20703 (2011).
Zhu, J., An, K. & Schleyer, P. v. R. Evaluation of triplet aromaticity by the isomerization stabilization energy. Org. Lett. 15, 2442–2445 (2013).
An, K. & Zhu, J. Evaluation of triplet aromaticity by the indene–isoindene isomerization stabilization energy method. Eur. J. Org. Chem. 13, 2764–2769 (2014).
Wan, P. & Krogh, E. Evidence for the generation of aromatic cationic systems in the excited state. Photochemical solvolysis of fluoren-9-ol. J. Chem. Soc. Chem. Commun. 1207–1208 (1985).
McAuley, I., Krogh, E. & Wan, P. Carbanion intermediates in the photodecarboxylation of benzannelated acetic acids in aqueous solution. J. Am. Chem. Soc. 110, 600–602 (1988).
Shukla, D. & Wan, P. Evidence for a planar cyclically conjugated 8π system in the excited state: large Stokes shift observed for dibenz[b,f]oxepin fluorescence. J. Am. Chem. Soc. 115, 2990–2991 (1993).
Wörner, H. J. & Merkt, F. Photoelectron spectroscopic study of the first singlet and triplet states of the cyclopentadienyl cation. Angew. Chem. Int. Ed. 45, 293–296 (2006).
Möllerstedt, H., Piqueras, M. C., Crespo, R. & Ottosson, H. Fulvenes, fulvalenes, and azulene: are they aromatic chameleons?. J. Am. Chem. Soc. 126, 13938–13939 (2004).
Ottosson, H. et al. Scope and limitations of Baird's theory on triplet state aromaticity: application to the tuning of singlet–triplet energy gaps in fulvenes. Chem. Eur. J. 13, 6998–7005 (2007).
Rosenberg, M., Ottosson, H. & Kilså, K. Influence of excited state aromaticity in the lowest excited singlet states of fulvene derivatives. Phys. Chem. Chem. Phys. 13, 12912–12919 (2011).
Jorner, K. et al. Impact of ground- and excited-state aromaticity on cyclopentadiene and silole excitation energies and excited-state polarities. Chem. Eur. J. 20, 9295–9303 (2014).
Rath, H. et al. Bis-rhodium hexaphyrins: metalation of [28]hexaphyrin and a smooth Hückel aromatic–antiaromatic interconversion. Chem. Commun. 3762–3764 (2009).
Stępień, M., Sprutta, N. & Latos-Grażyński, L. Figure eights, Möbius bands, and more: conformation and aromaticity of porphyrinoids. Angew. Chem. Int. Ed. 50, 4288–4340 (2011).
Saito, S. & Osuka, A. expanded porphyrins: intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 50, 4342–4373 (2011).
Shin, J.-Y. et al. Aromaticity and photophysical properties of various topology-controlled expanded porphyrins. Chem. Soc. Rev. 39, 2751–2767 (2010).
Cho, S. et al. Defining spectroscopic features of heteroannulenic antiaromatic porphyrinoids. J. Phys. Chem. Lett. 1, 895–900 (2010).
Kadish, K. M., Smith, K. M. & Guilard, R. Handbook of porphyrin science Vols 1–20 (World Scientific, 2010).
Ahn, T. K. et al. Comparative photophysics of [26]- and [28]Hexaphyrins(1.1.1.1.1.1): large two-photon absorption cross section of aromatic [26]hexaphyrins(1.1.1.1.1.1). J. Am. Chem. Soc. 127, 12856–12861 (2005).
Gouterman, M., Wagniere, G. H. & Snyder, L. C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 11, 108–127 (1963).
Schleyer, P. v. R., Maerker, C., Dransfeld, A., Jiao, H. & van Eikema Hommes, N. J. R. Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J. Am. Chem. Soc. 118, 6317–6318 (1996).
Geuenich, D., Hess, K., Köhler, F. & Herges, R. Anisotropy of the induced current density (acid), a general method to quantify and visualize electronic delocalization. Chem. Rev. 105, 3758–3772 (2005).
Bühl, M. et al. Helium and lithium NMR chemical shifts of endohedral fullerene compounds: an ab initio study. J. Am. Chem. Soc. 116, 6005–6006 (1994).
Krygowski, T. M. & Cyrański, M. K. Structural aspects of aromaticity. Chem. Rev. 101, 1385–1419 (2001).
Herges, R. Topology in chemistry: designing Möbius molecules. Chem. Rev. 106, 4820–4842 (2006).
Yoon, M.-C., Cho, S., Suzuki, M., Osuka, A. & Kim, D. Aromatic versus antiaromatic effect on photophysical properties of conformationally locked trans-vinylene-bridged hexaphyrins. J. Am. Chem. Soc. 131, 7360–7367 (2009).
Acknowledgements
This work at Yonsei University was supported by the Samsung Science and Technology Foundation (Project No. SSTF-BA1402-10). The quantum calculations were performed using the supercomputing resources of the Korea Institute of Science and Technology Information (KISTI). The work at Kyoto University was supported financially by the Global Research Laboratory (GRL, 2013K1A1A2A0205183) Program funded by the Ministry of Education, Science and Technology (MEST) of Korea. The authors express their appreciation to R. Herges and J.L. Sessler for valuable discussions.
Author information
Authors and Affiliations
Contributions
D.K. conceived and designed the experiments. Y.M.S., M.-C.Y. and J.M.L. performed the experiments. Y.M.S. and M.-C.Y. analysed the data. H.R., K.N. and A.O. contributed materials. Y.M.S., A.O. and D.K. co-wrote the paper. D.K. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2150 kb)
Rights and permissions
About this article
Cite this article
Sung, Y., Yoon, MC., Lim, J. et al. Reversal of Hückel (anti)aromaticity in the lowest triplet states of hexaphyrins and spectroscopic evidence for Baird's rule. Nature Chem 7, 418–422 (2015). https://doi.org/10.1038/nchem.2233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2233
This article is cited by
-
Symmetric carbon tetramers forming spin qubits in hexagonal boron nitride
npj Computational Materials (2023)
-
Baird’s rules at the tipping point
Nature Chemistry (2022)
-
Inversion of Aromaticity of NH-Tautomers of Free-Base Corroles in the Lowest Triplet T1-State
Journal of Applied Spectroscopy (2022)
-
Isolation of a triplet benzene dianion
Nature Chemistry (2021)
-
A flat carborane with multiple aromaticity beyond Wade–Mingos’ rules
Nature Communications (2020)