Abstract
Hedgehog (Hh) signalling is important in development, stem cell biology and disease. In a variety of tissues, Hh acts as a morphogen to regulate growth and cell fate specification. Several hypotheses have been proposed to explain morphogen movement, one of which is transport along filopodia-like protrusions called cytonemes. Here, we analyse the mechanism underlying Hh movement in the wing disc and the abdominal epidermis of Drosophila melanogaster. We show that, in both epithelia, cells generate cytonemes in regions of Hh signalling. These protrusions are actin-based and span several cell diameters. Various Hh signalling components localize to cytonemes, as well as to punctate structures that move along cytonemes and are probably exovesicles. Using in vivo imaging, we show that cytonemes are dynamic structures and that Hh gradient establishment correlates with cytoneme formation in space and time. Indeed, mutant conditions that affect cytoneme formation reduce both cytoneme length and Hh gradient length. Our results suggest that cytoneme-mediated Hh transport is the mechanistic basis for Hh gradient formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lawrence, P. A. Morphogens: how big is the big picture? Nat. Cell Biol. 3, E151–E154 (2001).
Gradilla, A. C. & Guerrero, I. Hedgehog on the move: a precise spatial control of Hedgehog dispersion shapes the gradient. Curr. Opin. Genet. Dev. 23, 363–373 (2013).
Crick, F. Diffusion in embryogenesis. Nature 225, 420–422 (1970).
Entchev, E. V., Schwabedissen, A. & Gonzalez-Gaitan, M. Gradient formation of the TGF-beta homolog Dpp. Cell 103, 981–991 (2000).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14, 1036–1045 (2012).
Greco, V., Hannus, M. & Eaton, S. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106, 633–645 (2001).
Panakova, D., Sprong, H., Marois, E., Thiele, C. & Eaton, S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435, 58–65 (2005).
Ramirez-Weber, F. A. & Kornberg, T. B. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97, 599–607 (1999).
Roy, S., Hsiung, F. & Kornberg, T. B. Specificity of Drosophila cytonemes for distinct signaling pathways. Science 332, 354–358 (2011).
Ingham, P. W., Nakano, Y. & Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 12, 393–406 (2011).
Porter, J. A., Young, K. E. & Beachy, P. A. Cholesterol modification of hedgehog signaling proteins in animal development. Science 274, 255–259 (1996).
Pepinsky, R. B. et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J. Biol. Chem. 273, 14037–14045 (1998).
Strigini, M. & Cohen, S. M. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development 124, 4697–4705 (1997).
Mullor, J. L., Calleja, M., Capdevila, J. & Guerrero, I. Hedgehog activity, independent of decapentaplegic, participates in wing disc patterning. Development 124, 1227–1237 (1997).
Garcia-Bellido, A., Ripoll, P. & Morata, G. Developmental compartmentalisation of the wing disk of Drosophila. Nat. New Biol. 245, 251–253 (1973).
Tabata, T. & Kornberg, T. B. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76, 89–102 (1994).
Porter, J. A. et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 374, 363–366 (1995).
Capdevila, J., Estrada, M. P., Sanchez-Herrero, E. & Guerrero, I. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 13, 71–82 (1994).
Chen, Y. & Struhl, G. Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563 (1996).
Struhl, G., Barbash, D. A. & Lawrence, P. A. Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development 124, 2143–2154 (1997).
Kopp, A., Muskavitch, M. A. & Duncan, I. The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development 124, 3703–3714 (1997).
Kopp, A. & Duncan, I. Anteroposterior patterning in adult abdominal segments of Drosophila. Dev. Biol. 242, 15–30 (2002).
Madhavan, M. M. & Madhavan, K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J. Embryol. Exp. Morphol. 60, 1–31 (1980).
Zheng, X., Mann, R. K., Sever, N. & Beachy, P. A. Genetic and biochemical definition of the Hedgehog receptor. Genes. Dev. 24, 57–71 (2010).
Yao, S., Lum, L. & Beachy, P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125, 343–357 (2006).
Yan, D. et al. The cell-surface proteins Dally-like and Ihog differentially regulate Hedgehog signaling strength and range during development. Development 137, 2033–2044 (2010).
Kim, M. S., Saunders, A. M., Hamaoka, B. Y., Beachy, P. A. & Leahy, D. J. Structure of the protein core of the glypican Dally-like and localization of a region important for hedgehog signaling. Proc. Natl Acad. Sci. USA 108, 13112–13117 (2011).
Callejo, A. et al. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc. Natl Acad. Sci. USA 108, 12591–12598 (2011).
Bloor, J. W. & Kiehart, D. P. zipper nonmuscle myosin-II functions downstream of PS2 integrin in Drosophila myogenesis and is necessary for myofibril formation. Dev. Biol. 239, 215–228 (2001).
Langhorst, M. F., Reuter, A. & Stuermer, C. A. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol. Life Sci. 62, 2228–2240 (2005).
Han, C., Jan, L. Y. & Jan, Y. N. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila. Proc. Natl Acad. Sci. USA 108, 9673–9678 (2011).
Bilioni, A. et al. Balancing Hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/DmWif. Dev. Biol. 376, 198–212 (2013).
Torroja, C., Gorfinkiel, N. & Guerrero, I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development 131, 2395–2408 (2004).
Burke, R. et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99, 803–815 (1999).
Lum, L. et al. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science 299, 2039–2045 (2003).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Mallavarapu, A. & Mitchison, T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J. Cell Biol. 146, 1097–1106 (1999).
Lee, K., Gallop, J. L., Rambani, K. & Kirschner, M. W. Self-assembly of filopodia-like structures on supported lipid bilayers. Science 329, 1341–1345 (2010).
Mavrakis, M., Rikhy, R. & Lippincott-Schwartz, J. Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Dev. Cell 16, 93–104 (2009).
Cooper, J. A. & Sept, D. New insights into mechanism and regulation of actin capping protein. Int. Rev. Cell Mol. Biol. 267, 183–206 (2008).
Delalle, I., Pfleger, C. M., Buff, E., Lueras, P. & Hariharan, I. K. Mutations in the Drosophila orthologs of the F-actin capping protein α- and β-subunits cause actin accumulation and subsequent retinal degeneration. Genetics 171, 1757–1765 (2005).
Uruno, T., Remmert, K. & Hammer, J. A. 3rd CARMIL is a potent capping protein antagonist: identification of a conserved CARMIL domain that inhibits the activity of capping protein and uncaps capped actin filaments. J. Biol. Chem. 281, 10635–10650 (2006).
Chhabra, E. S. & Higgs, H. N. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110–1121 (2007).
Kunda, P., Craig, G., Dominguez, V. & Baum, B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr. Biol. 13, 1867–1875 (2003).
Rogers, S. L., Wiedemann, U., Stuurman, N. & Vale, R. D. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 162, 1079–1088 (2003).
Georgiou, M. & Baum, B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J. Cell Sci. 123, 1089–1098 (2010).
Lyulcheva, E. et al. Drosophila pico and its mammalian ortholog lamellipodin activate serum response factor and promote cell proliferation. Dev. Cell 15, 680–690 (2008).
Neumann-Giesen, C. et al. Membrane and raft association of reggie-1/flotillin-2: role of myristoylation, palmitoylation and oligomerization and induction of filopodia by overexpression. Biochem. J. 378, 509–518 (2004).
Katanaev, V. L. et al. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. EMBO J. 27, 509–521 (2008).
Raposo, G. & Stoorvogel, W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383 (2013).
Rusten, T. E., Vaccari, T. & Stenmark, H. Shaping development with ESCRTs. Nat. Cell Biol. 14, 38–45 (2012).
Bellaiche, Y., The, I. & Perrimon, N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature 394, 85–88 (1998).
Takei, Y., Ozawa, Y., Sato, M., Watanabe, A. & Tabata, T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131, 73–82 (2004).
Tanaka, Y., Okada, Y. & Hirokawa, N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature 435, 172–177 (2005).
Sanders, T.A., Llagostera, E. & Barna, M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature 497, 628–632 (2013).
Cohen, M., Georgiou, M., Stevenson, N. L., Miodownik, M. & Baum, B. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev. Cell 19, 78–89 (2010).
Zeng, X. et al. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 (2001).
Lander, A. D., Nie, Q. & Wan, F. Y. Do morphogen gradients arise by diffusion? Dev. Cell 2, 785–796 (2002).
Saha, K. & Schaffer, D. V. Signal dynamics in Sonic hedgehog tissue patterning. Development 133, 889–900 (2006).
Hsiung, F., Ramirez-Weber, F. A., Iwaki, D. D. & Kornberg, T. B. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature 437, 560–563 (2005).
Sato, M. & Kornberg, T. B. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev. Cell 3, 195–207 (2002).
De Joussineau, C. et al. Delta-promoted filopodia mediate long-range lateral inhibition in Drosophila. Nature 426, 555–559 (2003).
Peng, Y., Han, C. & Axelrod, J. D. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev. Cell 23, 507–518.10.1016/j.devcel.2012.07.016
Rojas-Rios, P., Guerrero, I. & Gonzalez-Reyes, A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 10, e1001298 (2012).
Grigliatti, T. A., Hall, L., Rosenbluth, R. & Suzuki, D. T. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol. Gen. Genet. 120, 107–114 (1973).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of alteringcell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Han, C., Belenkaya, T. Y., Wang, B. & Lin, X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development 131, 601–611 (2004).
Moline, M. M., Southern, C. & Bejsovec, A. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo. Development 126, 4375–4384 (1999).
Millard, T. H. & Martin, P. Dynamic analysis of filopodial interactions duringthe zippering phase of Drosophila dorsal closure. Development 135, 621–626 (2008).
Roper, K., Mao, Y. & Brown, N. H. Contribution of sequence variation in Drosophila actins to their incorporation into actin-based structures in vivo. J. Cell Sci. 118, 3937–3948 (2005).
Pignoni, F. & Zipursky, S. L. Induction of Drosophila eye development by decapentaplegic. Development 124, 271–278 (1997).
Bischoff, M. & Cseresnyes, Z. Cell rearrangements, cell divisions and cell death in a migrating epithelial sheet in the abdomen of Drosophila. Development 136, 2403–2411 (2009).
R Development Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2011).
Acknowledgements
We are grateful to P. Lawrence in whose laboratory the abdomen work was done. We also thank T. Kornberg for helpful insights, V. Dietrich-Bischoff, P. Lawrence and C. Delidakis for helpful comments on the manuscript, T. Tabata for anti-Hh antibody, X. Lin for Dlp–GFP flies, BDSC and VDRC for stocks, R. Wilson for assisting with statistical analysis, C. Ibañez and V. Sánchez for helping with the RNAi screening, and A. Callejo, E. Mollica and A. Bilioni for initial observations. We also thank M. Guerra at the EM Unit and the confocal microscopy service of the Centro de Biologı´a Molecular ‘Severo Ochoa,’ as well as the confocal facility of the Department of Zoology—which is funded by the Wellcome Trust and the Isaac Newton Trust—for skillful technical assistance. Work was supported by grant BFU2011-25987 and Consolider Program CDS 2007-00008 from the Spanish MICINN, by Marie Curie FP7 (ITN 238186) and by an institutional grant from the Fundación Areces to the CBMSO. I.S. was supported by a FPI fellowship from the Spanish MICINN, A-C.G. by a Marie Curie ITN FP7 contract, G.A. by the Amarouto Program for senior researchers from the Comunidad Autónoma de Madrid and grant BFU2009-08085 from the Spanish MICINN, and M.B. by Project Grant WG086986 and Senior Investigator Award WT096645MA by the Wellcome Trust to P. Lawrence.
Author information
Authors and Affiliations
Contributions
M.B. designed and performed experiments, analysed data and wrote the paper; A-C.G. performed experiments and analysed data; I.S. and L.G-M. performed experiments; G.A. designed and performed experiments and analysed data; C.R-N. generated constructs for Flo2 overexpression and helped with RNAi screening and generating mutant clones; I.G. designed and performed experiments, analysed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
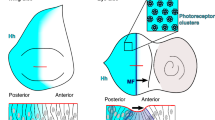
Supplementary Figure 1 (a,b) Hh signalling and cytonemes in the abdominal epidermis.
(a) Scheme depicting Hh and Ptc expression in the histoblasts and the larval epithelial cells (LECs). One segment (A and P compartment) with parts of the neighbouring segments shown. Anterior is to the left. Left panel: Histoblasts replace the LECs. Hh is expressed in the P compartment of histoblasts and LECs. In the A compartment, Ptc is expressed in a gradient in histoblasts and LECs (1) as well as in a row of LECs at the segment boundaries (2). Right panel: Histoblasts after all LECs have been replaced. Hh is expressed in the P compartment. In the A compartment, Ptc is expressed in a gradient (3) as well as in a narrow stripe at the segment boundaries (4). (b) We observe cytonemes only in areas of Hh signalling. In particular, cytonemes occur in areas where long-range signalling occurs (1,3). At the segment boundary (4), where only local signalling is occurring, no cytonemes can be observed. (c,d) The histoblast A/P compartment border is enriched with actin. All cells express the actin cytoskeleton marker Utrophin::GFP. (c) Apical plane of a z-stack, showing expanding histoblast nests after fusion. The cyan dotted line indicates the A/P border. In a cross section along the red line (red box), enrichment of actin at the A/P border can be seen (cyan arrow). (d) This actin enrichment becomes more obvious in a basal z-plane (white arrows). Bars, 10 μm. Anterior is to the left. (e,f) Cytonemes of P compartment cells are oriented anteriorly in the abdomen. Two examples of FLP-out clones that express UAS.Ihog-RFP to label cytonemes and UAS.RedStinger to label cell nuclei (e,f). Yellow arrows indicate cytonemes that point in anterior direction. Red arrows indicate the area posterior of the clones, where no cytonemes are present. Anterior is to the left.
Supplementary Figure 2 The shape of the Hh activity gradient correlates with the density of cytonemes along the a-p axis in wing discs.
(a) The top panel shows a merge of Hh activity gradient (Ptc-promotor-trap::GFP) and cytonemes labelled with Ihog-RFP. The bottom panel shows profile plots, which depict the vertically averaged pixel intensities along the horizontal axis of both gradient (green curve) and cytonemes (red curve). To merge both curves, the gradient curve was transformed along the y-axis to fit the maximum intensity of the cytoneme curve. Note that the shape of the Hh activity gradient and the density of cytonemes along the a-p axis correlate: The short array (cyan dotted line) coincides with the brightest section of the gradient; then both curves decline similarly. (b) Five additional examples of randomly chosen wing discs. Declining from the highest peak, both curves correlate (Spearman’s rank correlation coefficient (R) is shown).
Supplementary Figure 3 Analysis of the dynamic behaviour of cytonemes.
A video (Supplementary Video S10), in which cytonemes were labelled with GMA-GFP, was used to quantify the number of cytonemes per video frame (a) as well as the position of the cytonemes along the A/P boundary (b). All individual data points are shown. The video begins shortly after anterior and posterior histoblast nests have fused and continues for 15 hours. (a) Cytonemes that were distinguishable from the dense array (8 μm wide) were scored as ‘short’ when they were up to 8 μm long and as ‘long’ when they were longer than 8 μm. Numbers were plotted for each frame of the video (366 frames). We found, on average, 2.5±1.8 ‘long’ cytonemes per frame (n = 984) and 11.0±3.5 ‘short’ cytonemes per frame (n = 3301). (b) To score the position at the A/P boundary at which cytonemes are generated, we plotted the y-coordinates of each ‘long’ protrusion for each frame. This shows that, statistically, cytonemes form at any position along the A/P boundary throughout development. In the analysed video, the dense array of cytonemes is finally established (frame 61) around 2.5 hours after the anterior and posterior histoblast nests have fused completely (frame 13). During these 2.5 hours, the pattern of cytonemes along the y-axis differs from that later in development (b). Also the number of long cytonemes is steadily increasing over this period (a). These two hours of development correlate with the early stages of gradient establishment, which suggests that like in the experiments with Ihog-RFP overexpression (Supplementary Video S6), cytonemes first have to be established and that their establishment correlates with the establishment of the gradient.
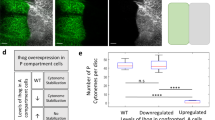
Supplementary Figure 4 Knock down of Cpa by RNAi leads to the accumulation of actin and myosin in wing imaginal disc cells.
Phalloidin was used to label actin, a ubiquitously expressed UAS.Myosin-Cherry was used to visualise myosin and α-Ptc to indicate the A/P boundary (n = 18 discs). Anterior is to the left. (a) Wild-type. Both A and P compartment cells have the same levels of actin in apical (a) and lateral (a’) regions of the disc. (a”) merge. (b) Driving UAS.Cpa-RNAi with hh.Gal4 in P compartment cells leads to an increase of actin in the P compartment, both in apical (b) and basal (b’) regions of the disc. (b”) merge. (c) In wild-type, myosin levels are comparable in A and P compartment cells. (d) Driving UAS.Cpa-RNAi with hh.Gal4 in the P compartment leads to an increase of myosin levels in basal regions of the P compartment. (d’) merge.
Supplementary Figure 5 Analysing the Hh activity gradient without the overexpression of Ihog.
(a-f) Manipulating Cpa, Scar, Pico and Flo2 levels in P compartment cells of wing discs affects the Hh activity gradient. (a) Control comprising Ptc-promotor-trap::GFP only. (b) Cpa-RNAi. (c) Scar-RNAi. (d) Pico-RNAi. (e) UAS.Flo2. (f) Flo2-RNAi. Left panels: The Hh activity gradient is visualised using Ptc-promotor-trap::GFP. Right panels: Quantification of gradient length in the wing discs shown in the left panels using profile plots. The graphs depict the vertically averaged pixel intensities along the horizontal (a–p) axis of the detail of the Ptc-promotor-trap::GFP image shown in the top left corner. The red arrows indicate the length of the gradient, up to the point where the average pixel intensity drops below 5% of the maximum intensity. In the RNAi wing discs (b-d,f), the gradient is shorter than in the control (a). In UAS.Flo2 wing discs (e), the gradient is longer than in the control (a). In all images, anterior is to the left. (g) In the abdomen, the Ptc-promotor-trap::GFP gradient is shorter when Ihog-RFP is co-expressed. Quantification of the Hh activity gradient in Ptc-promotor-trap::GFP pupae as well as in UAS.Ihog-RFP, Ptc-promotor-trap::GFP pupae using profile plots. These plots show the vertically averaged pixel intensities along the horizontal axes of Ptc-promotor-trap::GFP images of a standardised area (149 μm×35 μm) covering the gradient and the A/P compartment border at 12 hours after the ADH and PDH nests fuse. Graphs, each representing a different individual pupa, were aligned relative to the x-axis (i.e. the image’s a-p axis) using the A/P border (blue arrow). Pixel intensities vary between different individuals due to different imaging conditions. This is, however, irrelevant for our analysis, since we are focusing on the changes in fluorescence along the x-axis in each individual. In Ptc-promotor-trap::GFPpupae, there are three rows of cells that have similarly high levels of fluorescence close to the A/P border (asterisks), whereas in UAS.Ihog-RFP, Ptc-promotor-trap::GFP individuals, only one row of cells shows high fluorescence levels (asterisk). Interestingly, the first row of bristle precursors is positioned immediately anterior to the highest levels of fluorescence, as indicated by the hatched line. In the Ptc-promotor-trap::GFP pupae, these precursors lie approximately two cell diameters more anterior than in the UAS.Ihog-RFP, Ptc-promotor-trap::GFP pupae. This observation is consistent with the notion that the Hh activity gradient is extended, since the specification of bristle precursors is dependent on Hh signalling. A shortening of the gradient should therefore ‘move’ bristles posteriorly. Anterior is to the left.
Supplementary Figure 6 Hh protein levels are not affected by the manipulation of cytoneme length.
Either the expression of CPa-RNAi (a), Scar-RNAi (b), Pico-RNAi (c), UAS.Flo2 (d) or Flo2-RNAi (e) in the dorsal compartment of wing discs using ap.Gal4 affects Hh protein levels. Hh levels are similar in the mutant (dorsal) and the control (ventral) parts of the P compartment after 24 hours of expression. GFP is co-expressed to label the dorsal compartment, and α-Hh labels the P compartment. A hatched line indicates the D/V border. n∼8 discs per treatment. Anterior is to the left.
Supplementary Figure 7 Interfering with exovesicle production/release affects the length of the Hh activity gradient.
(a–d) Fluorescence images of wing discs, in which the Hh activity gradient in A compartment cells is visualised using the Ptc-promotor-trap::GFP reporter. A magnification of the Ptc signal is shown at the bottom. RNAi-constructs were expressed in the P compartment using hh.Gal4. (a) Wild-type control. (b) Alix-RNAi. (c) TSG101-RNAi. (d) HRS-RNAi. In the RNAi wing discs (b-d), the gradients are shorter than in the control (a). In all images, anterior is to the left. (e) Box plot comparing gradient length between control discs and the RNAi treatments. There were no significant differences among the five RNAi treatments, but a significant difference between the control and each of the six RNAi treatments. See Methods for details on the statistical analysis.
Supplementary Figure 8 Cytonemes play a role in Hh transport—scheme of possible cytoneme function.
Our data suggest that Hh is transported from the sending cells along cytonemes to the receiving cells. Cytoneme length correlates with gradient length, suggesting that this transport is crucial for the establishment of a gradient of normal length. Since cytonemes are basal structures, this indicates that the Hh activity gradient is formed basally. Using Ihog-RFP, we observed punctate structures moving in both directions along cytonemes. Our results suggest that these puncta could be exovesicles, We also noticed puncta leaving the cytonemes. It is therefore possible that after transport, vesicles can leave cytonemes to reach targets further away from the Hh source. In the wing disc, as is shown in the scheme, the receiving cells also produce cytonemes that orient towards the sending cells. Thus, it is possible that sending and receiving cytonemes directly exchange Hh. Another possibility could be that Hh leaves the sending cytonemes before being sequestered by the receiving cytonemes. The observation that cytonemes are very dynamic structures suggests that they do not serve as stable conduits but rather transport some cargo quickly and then retract. The retraction could be triggered by contact with a receiving cytoneme/cell body or by a cytoneme-intrinsic mechanism due to actin filament instability.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1871 kb)
Histoblasts of the P compartment display cytonemes.
These protrusions are basal and reach far into the A compartment, across the entire length of the Ptc-promotor-trap::GFP gradient. Scan through a z-stack from apical to basal is shown. Cells of the P compartment are labelled with hh.Gal4>UAS.Ihog-RFP (red). In addition, Hh responding cells in the A compartment are marked with Ptc-promotor-trap::GFP (green). Note a dense array of shorter protrusions and individual longer protrusions. White line indicates A/P border. Bar, 10 μm; z-step size, 2.5 μm; around 31 h APF. See also Fig. 4c. (MOV 1578 kb)
Development of cytonemes and Hh signalling gradient in the abdomen.
P compartment cells are labelled with hh.Gal4>UAS.Ihog-RFP. In the A compartment, Ptc-promotor-trap::GFP labels cells responding to Hh signalling. Four panels are shown: (1) Ihog-RFP apical sections, (2) Ihog-RFP basal sections, (3) Ptc-promotor-trap::GFP and (4) merge of Ihog-RFP and Ptc-promotor-trap::GFP channels. At the beginning of the video, the ADH and the PDH nest are still separated by a row of LECs (asterisks in ‘merge’). At this time, no cytonemes are present but some Ptc-promotor-trap::GFP expression is already visible. Once the two nests start to fuse (indicated by arrow in ‘merge’), cytonemes start to form basally and the Ptc gradient becomes established. A red arrow in the top right panel points towards a long cytoneme towards the end of the video. Note that the start of Ptc-promotor-trap::GFP expression in one row of A compartment LECs, which are located posterior to the P compartment, coincides with the generation of bundles of cytonemes by the P compartment LECs (Fig. 4f). Bar, 20 μm; video begins at around 16 h APF; time interval between frames, 2.5 min. (MOV 14824 kb)
Histoblasts of the A compartment only rarely produce short cytonemes that point into the P compartment.
Cells are labelled with ptc.Gal4>UAS.Ihog-CFP. Orange line indicates dorsal midline. A/P border of the left hemisegment of segment A2 indicated by white line at the beginning of video. Bar, 20 μm; video begins at around 38 h APF; time interval between frames, 2.5 min. (MOV 4148 kb)
LECs of the P compartment produce cytonemes during morphogenesis.
These protrusions mainly point in anterior (arrows), but also in posterior direction (arrowhead). LECs are labelled with hh.Gal4>UAS.Ihog-CFP. Bar, 20 μm; video begins at around 25 h APF; time interval between frames, 2.5 min. See Fig. 4e. (MOV 8638 kb)
LECs of the P compartment produce cytonemes at the segment (P/A) border, just when morphogenesis begins.
These protrusions ‘surround’ the first row of LECs of the A compartment (asterisks). At the tip of the protrusions, vesicles emanate (arrow). Cells are labelled with hh. Gal4>UAS.Ihog-RFP. Bar, 20 μm; video begins at around 15 h APF; time interval between frames, 2.5 min. See Fig. 4f. (MOV 3801 kb)
Cytoneme development correlates with gradient formation.
hh.Gal4>UAS.Ihog-RFP labels P cytonemes, and Ptc-promotor-trap::GFP marks Hh signalling gradient. Top panel: Ptc-promotor-trap::GFP channel. Middle panel: the graph shows the vertically averaged pixel intensities along the horizontal axis of the panel above (Ptc-promotor-trap::GFP channel). Bottom panel: basal section of the Ihog-RFP channel displayed using an inverted grey-scale lookup table. At the beginning of the video, the ADH and PDH nests are still separated by a row of LECs (asterisk). Once the two nests meet, a red line appears and then moves posteriorly, indicating the length of the longest cytoneme. The growth of the cytonemes correlates with the widening of the gradient, which can be seen in the graph. The most posterior row of GFP-positive cells in the top panel lie at the A/P border; the border is also indicated by a blue line in the last frame of the video. Note that cytonemes only appear after the ADH and PDH nests have fused, however, a faint GFP signal is present before (green arrows). At the end of the video, the cytonemes are present and the gradient is established. Maximum cytoneme length (red line) correlates with the width of the gradient. All cytonemes that are visible in the shown basal section reach into the A compartment. A dense network of shorter cytonemes and longer individual cytonemes are present. Bar, 10 μm; video begins around 16 h APF; time interval between frames, 2.5 min. Panels taken from Supplementary Fig. S2. (MOV 14473 kb)
The shape of the Hh activity gradient and the density of cytonemes along the a-p axis correlate throughout development.
Part of Supplementary Video S6 with the addition of the cytoneme profile plot. hh.Gal4>UAS.Ihog-RFP labels P cytonemes, and Ptc-promotor-trap::GFP marks the Hh signalling gradient. Top panel: Ptc-promotor-trap::GFP channel. Middle panel: the curves depict the vertically averaged pixel intensities along the horizontal axis of both the panel above (green curve) and below (red curve). Bottom panel: basal section of the Ihog-RFP channel displayed using an inverted grey-scale look-up table. The video starts when ADH and PDH nests have just met. The red line indicates the length of the longest cytoneme. The growth of the cytonemes correlates with the widening of the gradient, which can be seen in the green curve. The most posterior row of GFP-positive cells in the top panel lies at the A/P border; in some frames of the video, the border is also indicated by a blue line. At the end of the video, cytonemes are present and the gradient is established. Interestingly, the shape of the Hh activity gradient (green curve) and the density of cytonemes along the a-p axis (red curve) correlate: The short array (cyan line) coincides with the brightest section of the gradient; then both curves decline similarly (cyan arrow). Note that this relation is already visible while the gradient is forming. Video begins around 18 h APF; time interval between frames, 2.5 min. See Fig. 5d. (MOV 9486 kb)
Cytonemes of histoblasts of the P compartment labelled with the actin cytoskeleton marker GMA.
With this marker, cytonemes are more dynamic compared to cytonemes labelled with Ihog-RFP or Ihog-CFP (e.g. Supplementary Videos S6 and S14). LECs are labelled with hh.Gal4>UAS.GMA. Inverted grey-scale LUT. Bar, 10 μm; video begins at around 39 h APF; time interval between frames, 15 s. (MOV 8013 kb)
Cytonemes are highly dynamic.
High magnification, short time interval video of cytonemes of P compartment cells using the actin cytoskeleton marker GMA. Cells are marked with hh.Gal4>UAS.GMA and move down towards the dorsal midline, which is outside the image. Grey-scale LUT. Bar, 10 μm; video begins at around 36 h APF; time interval between frames, 7 s. See Fig. 5h. (MOV 20778 kb)
Analysis of the dynamic behaviour of cytonemes (see Supplementary Fig. S3).
Cytonemes of P compartment cells are labelled with hh.Gal4>UAS.GMA. At the beginning of the video, the anterior and posterior histoblast nests have already started to fuse. At frame 13, the fusion is complete and at frame 61, the short cytoneme array (red line) is fully established; the blue line indicates the A/P boundary. Merge of red (most apical slice of z-stack) and green (all other slices) indicates the location of the A/P boundary throughout the video. Video begins at around 19 h APF; time interval between frames, 2.5 min. (MOV 20144 kb)
CD4-Tomato labels cytonemes in the abdomen.
P compartment cells and their cytonemes are labelled with hh.Gal4>UAS.CD4-Tomato. Note that this membrane marker also labels puncta that emanate from cytonemes (black arrow). Inverted Fire Lookup table. Video begins at around 19 h APF; time interval between frames, 2.5 min. (MOV 9268 kb)
Abdominal cytonemes are labelled with the membrane marker Gap43-Venus. hh. Gal4 driving UAS.gap43-Venus41.
Inverted grey-scale LUT. Bar, 10 μm; time interval between frames, 14 s. (MOV 10864 kb)
Establishment of the Hh activity gradient in the abdomen in the absence of Ihog-RFP.
Top panel: Hh activity gradient labelled with Ptc-promotor-trap::GFP. Bottom panel: P compartment cells and their cytonemes are labelled with hh.Gal4>UAS.CD4-Tomato. The most apical section of the projected z-stack is coloured in red, thus indicating the compartment border (blue line in first frame). The cytonemes are coloured in black in front of a green background. Middle panel: the graph depicts the vertically averaged pixel intensities along the horizontal axis of the above panel (Hh activity gradient). The red line indicates the length of the longest cytoneme and the yellow line the length of the short array of cytonemes. At the beginning of the video, the ADH and PDH nests have already fused. Note that at the end of the video, the short array of cytonemes coincides with the peak of the Hh activity gradient. The longest cytonemes (red line) cover most of the Hh activity gradient. Video begins at around 22 h APF; time interval between frames, 2.5 min. (MOV 9086 kb)
Ihog-RFP puncta move along cytonemes.
A red arrow points at an individual cytoneme. Note that cytonemes are more stable compared to cytonemes that are labelled with GMA (Supplementary Videos S8– S10). Cells of the P compartment are labelled with hh.Gal4>UAS.Ihog-RFP. Bar, 5 μm; video begins at around 39 h APF; time interval between frames, 20 s. (MOV 6137 kb)
Ihog-RFP puncta emanate from the tips of cytonemes.
P compartment cells and their cytonemes are labelled with hh.Gal4>UAS.Ihog-RFP. Note that Ihog-RFP also labels puncta that are not associated to cytonemes. Yellow arrow points at an emanation event. Bar, 10 μm; video begins at around 25 h APF; time interval between frames, 2.5 min. (MOV 5715 kb)
Rights and permissions
About this article
Cite this article
Bischoff, M., Gradilla, AC., Seijo, I. et al. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol 15, 1269–1281 (2013). https://doi.org/10.1038/ncb2856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2856
This article is cited by
-
Cytoneme-mediated transport of active Wnt5b–Ror2 complexes in zebrafish
Nature (2024)
-
GPI-anchored FGF directs cytoneme-mediated bidirectional contacts to regulate its tissue-specific dispersion
Nature Communications (2022)
-
Cytonemes coordinate asymmetric signaling and organization in the Drosophila muscle progenitor niche
Nature Communications (2022)
-
Predictive model for cytoneme guidance in Hedgehog signaling based on Ihog- Glypicans interaction
Nature Communications (2022)
-
Hedgehog signaling and its molecular perspective with cholesterol: a comprehensive review
Cellular and Molecular Life Sciences (2022)