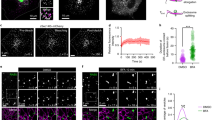
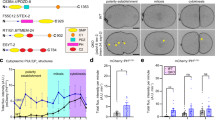
Abstract
Large pleiomorphic carriers leave the Golgi complex for the plasma membrane by en bloc extrusion of specialized tubular domains, which then undergo fission. Several components of the underlying molecular machinery have been identified, including those involved in the budding/initiation of tubular carrier precursors (for example, the phosphoinositide kinase PI(4)KIIIβ, the GTPase ARF, and FAPP2), and in the fission of these precursors (for example, PKD, CtBP1-S/BARS). However, how these proteins interact to bring about carrier formation is poorly understood. Here, we describe a protein complex that mediates carrier formation and contains budding and fission molecules, as well as other molecules, such as the adaptor protein 14-3-3γ. Specifically, we show that 14-3-3γ dimers bridge CtBP1-S/BARS with PI(4)KIIIβ, and that the resulting complex is stabilized by phosphorylation by PKD and PAK. Disrupting the association of these proteins inhibits the fission of elongating carrier precursors, indicating that this complex couples the carrier budding and fission processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
De Matteis, M. A. & Luini, A. Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 9, 273–284 (2008).
Hirschberg, K. et al. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 143, 1485–1503 (1998).
Polishchuk, E. V., Di Pentima, A., Luini, A. & Polishchuk, R. S. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol. Biol. Cell 14, 4470–4485 (2003).
Rodriguez-Boulan, E., Kreitzer, G. & Musch, A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247 (2005).
Rothman, J. E. Lasker Basic Medical Research Award. The machinery and principles of vesicle transport in the cell. Nat. Med. 8, 1059–1062 (2002).
Schekman, R. Lasker Basic Medical Research Award. SEC mutants and the secretory apparatus. Nat. Med. 8, 1055–1058 (2002).
Haynes, L. P., Thomas, G. M. & Burgoyne, R. D. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase β and trans-Golgi network–plasma membrane traffic. J. Biol. Chem. 280, 6047–6054 (2005).
Bruns, J. R., Ellis, M. A., Jeromin, A. & Weisz, O. A. Multiple roles for phosphatidylinositol 4-kinase in biosynthetic transport in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 277, 2012–2018 (2002).
Godi, A. et al. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 6, 393–404 (2004).
Godi, A. et al. ARF mediates recruitment of PtdIns-4-OH kinase- β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1, 280–287 (1999).
D’Angelo, G. et al. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449, 62–67 (2007).
Cao, X. et al. Golgi protein FAPP2 tubulates membranes. Proc. Natl Acad. Sci. USA 106, 21121–21125 (2009).
Diaz Anel, A. M. Phospholipase C β3 is a key component in the Gβγ/PKCη/PKD-mediated regulation of trans-Golgi network to plasma membrane transport. Biochem. J. 406, 157–165 (2007).
Liljedahl, M. et al. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104, 409–420 (2001).
Yeaman, C. et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 6, 106–112 (2004).
Miserey-Lenkei, S. et al. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 12, 645–654 (2010).
Bonazzi, M. et al. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7, 570–580 (2005).
Nardini, M. et al. CtBP/BARS: a dual-function protein involved in transcription co-repression and Golgi membrane fission. EMBO J. 22, 3122–3130 (2003).
Corda, D., Colanzi, A. & Luini, A. The multiple activities of CtBP/BARS proteins: the Golgi view. Trends Cell Biol. 16, 167–173 (2006).
Colanzi, A. & Corda, D. Mitosis controls the Golgi and the Golgi controls mitosis. Curr. Opin. Cell Biol. 19, 386–393 (2007).
Aitken, A. 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 16, 162–172 (2006).
Hausser, A. et al. Phospho-specific binding of 14-3-3 proteins to phosphatidylinositol 4-kinase III β protects from dephosphorylation and stabilises lipid kinase activity. J. Cell Sci. 119, 3613–3621 (2006).
Demmel, L. et al. Nucleocytoplasmic shuttling of the Golgi phosphatidylinositol 4-kinase Pik1 is regulated by 14-3-3 proteins and coordinates Golgi function with cell growth. Mol. Biol. Cell 19, 1046–1061 (2008).
Valente, C., Spanò, S., Luini, A. & Corda, D. Purification and functional properties of the membrane fissioning protein CtBP3/BARS. Methods Enzymol. 404, 296–316 (2005).
Zhao, X. et al. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase β stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J. Biol. Chem. 276, 40183–40189 (2001).
Hausser, A. et al. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase III β at the Golgi complex. Nat. Cell Biol. 7, 880–886 (2005).
Eswaran, J., Soundararajan, M., Kumar, R. & Knapp, S. UnPAKing the class differences among p21-activated kinases. Trends Biochem. Sci. 33, 394–403 (2008).
Rodriguez Boulan, E. & Pendergast, M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell 20, 45–54 (1980).
Matlin, K. S. & Simons, K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34, 233–243 (1983).
Polishchuk, R., Di Pentima, A. & Lippincott-Schwartz, J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 6, 297–307 (2004).
Marra, P. et al. The GM130 and GRASP65 Golgi proteins cycle through and define a subdomain of the intermediate compartment. Nat. Cell Biol. 3, 1101–1113 (2001).
Pelkmans, L. & Helenius, A. Insider information: what viruses tell us about endocytosis. Curr. Opin. Cell Biol. 15, 414–422 (2003).
Steinacker, P. et al. Unchanged survival rates of 14-3-3γ knockout mice after inoculation with pathological prion protein. Mol. Cell Biol. 25, 1339–1346 (2005).
Chaudhri, M., Scarabel, M. & Aitken, A. Mammalian and yeast 14-3-3 isoforms form distinct patterns of dimers in vivo. Biochem. Biophys. Res. Commun. 300, 679–685 (2003).
Yaffe, M. B. et al. The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91, 961–971 (1997).
Braselmann, S. & McCormick, F. Bcr and Raf form a complex in vivo via 14-3-3 proteins. EMBO J. 14, 4839–4848 (1995).
Vincenz, C. & Dixit, V. M. 14-3-3 proteins associate with A20 in an isoform-specific manner and function both as chaperone and adapter molecules. J. Biol. Chem. 271, 20029–20034 (1996).
Van Der Hoeven, P. C., Van Der Wal, J. C., Ruurs, P., Van Dijk, M. C. & Van Blitterswijk, J. 14-3-3 isotypes facilitate coupling of protein kinase C- ζ to Raf-1: negative regulation by 14-3-3 phosphorylation. Biochem. J. 345 Pt 2, 297–306 (2000).
Liberali, P. et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27, 970–981 (2008).
Liu, D. et al. Crystal structure of the ζ isoform of the 14-3-3 protein. Nature 376, 191–194 (1995).
Ottmann, C. et al. Phosphorylation-independent interaction between 14-3-3 and exoenzyme S: from structure to pathogenesis. EMBO J. 26, 902–913 (2007).
Barnes, C. J. et al. Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat. Struct. Biol. 10, 622–628 (2003).
Dharmawardhane, S. et al. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11, 3341–3352 (2000).
Paglini, G., Peris, L., Diez-Guerra, J., Quiroga, S. & Caceres, A. The Cdk5-p35 kinase associates with the Golgi apparatus and regulates membrane traffic. EMBO Rep. 2, 1139–1144 (2001).
Deacon, S. W. et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 15, 322–331 (2008).
Yang, J. S. et al. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 24, 4133–4143 (2005).
Yang, J. S. et al. COPI acts in both vesicular and tubular transport. Nat. Cell Biol. 13, 996–1003 (2011).
Hidalgo Carcedo, C. et al. Mitotic Golgi partitioning is driven by the membrane-fissioning protein CtBP3/BARS. Science 305, 93–96 (2004).
Colanzi, A. et al. The Golgi mitotic checkpoint is controlled by BARS-dependent fission of the Golgi ribbon into separate stacks in G2. EMBO J. 26, 2465–2476 (2007).
Saeed, M. F., Kolokoltsov, A. A., Albrecht, T. & Davey, R. A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 6, e1001110 (2010).
Haga, Y., Miwa, N., Jahangeer, S., Okada, T. & Nakamura, S. CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis. EMBO J. 28, 1197–1207 (2009).
Moreau, D. et al. Genome-wide RNAi screens identify genes required for ricin and PE intoxications. Dev. Cell 21, 231–244 (2011).
Tzivion, G. & Avruch, J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J. Biol. Chem. 277, 3061–3064 (2002).
Ponsioen, B. et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 5, 1176–1180 (2004).
Pusapati, G. V. et al. Role of the second cysteine-rich domain and Pro275 in PKD2 interaction with ARF1, TGN recruitment and protein transport. Mol. Biol. Cell 21, 1011–1022 (2010).
Beck, R. et al. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc. Natl Acad. Sci. USA 105, 11731–11736 (2008).
Krauss, M. et al. Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding. J. Biol. Chem. 283, 27717–27723 (2008).
Lundmark, R., Doherty, G. J., Vallis, Y., Peter, B. J. & McMahon, H. T. Arf family GTP loading is activated by, and generates, positive membrane curvature. Biochem. J. 414, 189–194 (2008).
Vieira, O. V., Verkade, P., Manninen, A. & Simons, K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J. Cell Biol. 170, 521–526 (2005).
Schmidt, J. A. & Brown, W. J. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell Biol. 186, 211–218 (2009).
Schwarz, K., Natarajan, S., Kassas, N., Vitale, N. & Schmitz, F. The synaptic ribbon is a site of phosphatidic acid generation in ribbon synapses. J. Neurosci. 31, 15996–16011 (2011).
Weigert, R. et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 402, 429–433 (1999).
Gallop, J. L., Butler, P. J. & McMahon, H. T. Endophilin and CtBP/BARSare not acyl transferases in endocytosis or Golgi fission. Nature 438, 675–678 (2005).
Pulvirenti, T. et al. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 10, 912–922 (2008).
Harlow, E. & Lane, D. Antibodies: A Laboratory Manual (CSH Press, 1988).
Malhotra, V., Serafini, T., Orci, L., Shepherd, J. C. & Rothman, J. E. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell 58, 329–336 (1989).
Leelavathi, D. E., Estes, L. V., Feingold, D. S. & Lombardi, B. Isolation of a Golgi rich fraction from rat liver. BBA 211, 124–138 (1970).
Verdoodt, B., Benzinger, A., Popowicz, G. M., Holak, T. A. & Hermeking, H. Characterization of 14-3-3σ dimerization determinants: requirement of homodimerization for inhibition of cell proliferation. Cell Cycle 5, 2920–2926 (2006).
Zhou, Y., Reddy, S., Murrey, H., Fei, H. & Levitan, I. B. Monomeric 14-3-3 protein is sufficient to modulate the activity of the Drosophila slowpoke calcium-dependent potassium channel. J. Biol. Chem. 278, 10073–10080 (2003).
Keller, P., Toomre, D., Diaz, E., White, J. & Simons, K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat. Cell Biol. 3, 140–149 (2001).
Acknowledgements
The authors would like to thank all colleagues who kindly provided them with antibodies and reagents (as listed under ‘Reagents’); M. A. De Matteis and C. Wilson for critical reading of the manuscript; J. Chernoff for the PAK inhibitor IPA-3 (Fox Chase Cancer Center); C. P. Berrie for critical reading of and editorial assistance with the manuscript; C. Cericola for preparation of the anti-BARS antibody; R. Le Donne and E. Fontana for preparation of the figures; the Integrated Microscopy Facility at the Institute of Genetics and Biophysics, National Research Council, Naples, and the Dynamic Imaging Microscopy Facility at the CEINGE Institute, Naples, for support in imaging microscopy, data processing and analysis; the Italian Association for Cancer Research (to D.C. IG4664 and IG10341, to A.L. IG4700, to A.C. IG6074 and to R.S.P. IG10233), Telethon Italia (to D.C. GGPO9274, to A.L. GGPO8231 and to R.S.P. GTF08001), the European Community Seventh Framework Programme FP7/2007-2013 HEALTH-F2-2007-201804 (Eucilia to A.L.), grant FIT DM 24/09/2009, Legge 46/82, and FaReBio, Ministry of Economy and Finance (to D.C.) for financial support. C.V., A.P. and S.S. were recipients of Italian Foundation for Cancer Research Fellowships (FIRC, Milan, Italy).
Author information
Authors and Affiliations
Contributions
C.V. designed, carried out and analysed all of the experiments and co-wrote the manuscript. G.T. carried out immunofluorescence and electron microscopy experiments. A.P. carried out COPI retrograde transport and macropinocytosis assays. G.D.T., M.S. and D.P. produced essential antibodies. F.F. carried out FRET and FLIM experiments. S.S. carried out initial experiments. R.G. carried out some phosphorylation experiments. R.S.P. carried out time-lapse microscopy. A.C. and S.M. contributed to relevant discussions and suggestions. D.C. and A.L. conceived and supervised the project, discussed and analysed the data and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 955 kb)
Supplementary Table 1
Supplementary Information (XLS 32 kb)
Supplementary Table 2
Supplementary Information (XLS 28 kb)
Supplementary Movie 1
Supplementary Information (MOV 2591 kb)
Supplementary Movie 2
Supplementary Information (MOV 2294 kb)
Supplementary Movie 3
Supplementary Information (MOV 8047 kb)
Supplementary Movie 4
Supplementary Information (MOV 2011 kb)
Supplementary Movie 5
Supplementary Information (MOV 611 kb)
Rights and permissions
About this article
Cite this article
Valente, C., Turacchio, G., Mariggiò, S. et al. A 14-3-3γ dimer-based scaffold bridges CtBP1-S/BARS to PI(4)KIIIβ to regulate post-Golgi carrier formation. Nat Cell Biol 14, 343–354 (2012). https://doi.org/10.1038/ncb2445
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2445
This article is cited by
-
Identification and characterization of a new potent inhibitor targeting CtBP1/BARS in melanoma cells
Journal of Experimental & Clinical Cancer Research (2024)
-
Phosphoinositides as membrane organizers
Nature Reviews Molecular Cell Biology (2022)
-
Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions
Nature Communications (2017)
-
PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions
Scientific Reports (2017)
-
Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase δ
Nature Communications (2016)