Abstract
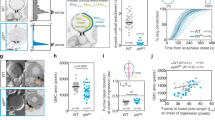
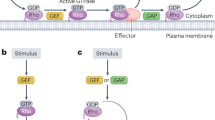
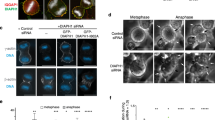
In animal cells, cytokinesis is powered by a contractile ring of actin filaments (F-actin) and myosin-2. Formation of the contractile ring is dependent on the small GTPase RhoA1,2, which is activated in a precise zone at the cell equator3. It has long been assumed that cytokinesis and other Rho-dependent processes are controlled in a sequential manner, whereby Rho activation by guanine nucleotide exchange factors (GEFs) initiates a particular event, and Rho inactivation by GTPase activating proteins (GAPs) terminates that event. MgcRacGAP is a conserved cytokinesis regulator thought to be required only at the end of cytokinesis4,5. Here we show that GAP activity of MgcRacGAP is necessary early during cytokinesis for the formation and maintenance of the Rho activity zone. Disruption of GAP activity by point mutation results in poorly focused Rho activity zones, whereas complete removal of the GAP domain results in unfocused zones that show lateral instability and/or rapid side-to-side oscillations. We propose that the GAP domain of MgcRacGAP has two unexpected roles throughout cytokinesis: first, it transiently anchors active Rho, and second, it promotes local Rho inactivation, resulting in the constant flux of Rho through the GTPase cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kishi, K., Sasaki, T., Kuroda, S., Itoh, T. & Takai, Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J. Cell Biol. 120, 1187–1195 (1993).
Drechsel, D. N., Hyman, A. A., Hall, A. & Glotzer, M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 7, 12–23 (1997).
Bement, W. M., Benink, H. A. & von Dassow, G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol. 170, 91–101 (2005).
Hirose, K., Kawashima, T., Iwamoto, I., Nosaka, T. & Kitamura, T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J. Biol. Chem. 276, 5821–5828 (2001).
Jantsch-Plunger, V. et al. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. J. Cell Biol. 149, 1391–1404 (2000).
Mishima, M., Kaitna, S. & Glotzer, M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2, 41–54 (2002).
Yuce, O., Piekny, A. & Glotzer, M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 (2005).
Somers, W. G. & Saint, R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39 (2003).
Minoshima, Y. et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev. Cell 4, 549–560 (2003).
Bement, W. M., Miller, A. L. & von Dassow, G. Rho GTPase activity zones and transient contractile arrays. Bioessays 28, 983–993 (2006).
Zavortink, M., Contreras, N., Addy, T., Bejsovec, A. & Saint, R. Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. J. Cell Sci. 118, 5381–92 (2005).
Lee, J. S., Kamijo, K., Ohara, N., Kitamura, T. & Miki, T. MgcRacGAP regulates cortical activity through RhoA during cytokinesis. Exp. Cell Res. 293, 275–282 (2004).
Yamada, T., Hikida, M. & Kurosaki, T. Regulation of cytokinesis by mgcRacGAP in B lymphocytes is independent of GAP activity. Exp. Cell Res. 312, 3517–25 (2006).
Goldstein, A. Y., Jan, Y. N. & Luo, L. Function and regulation of Tumbleweed (RacGAP50C) in neuroblast proliferation and neuronal morphogenesis. Proc. Natl Acad. Sci. USA 102, 3834–3839 (2005).
Gregory, S. L. et al. Cell division requires a direct link between microtubule-bound RacGAP and Anillin in the contractile ring. Curr. Biol. 18, 25–9 (2008).
Ban, R., Irino, Y., Fukami, K. & Tanaka, H. Human mitotic spindle-associated protein PRC1 inhibits MgcRacGAP activity toward Cdc42 during the metaphase. J. Biol. Chem. 279, 16394–16402 (2004).
Verbrugghe, K. J. & White, J. G. SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 14, 1755–1760 (2004).
Benink, H. A. & Bement, W. M. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–39 (2005).
Danilchik, M. V. & Brown, E. E. Membrane dynamics of cleavage furrow closure in Xenopus laevis. Dev. Dyn. 237, 565–579 (2008).
Birkenfeld, J. et al. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell 12, 699–712 (2007).
Zhang, B., Chernoff, J. & Zheng, Y. Interaction of Rac1 with GTPase-activating proteins and putative effectors. A comparison with Cdc42 and RhoA. J. Biol. Chem. 273, 8776–82 (1998).
Cho, Y. J. et al. Abr and Bcr, two homologous Rac GTPase-activating proteins, control multiple cellular functions of murine macrophages. Mol. Cell Biol. 27, 899–911 (2007).
Fu, Y. & Galan, J. E. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401, 293–297 (1999).
Leonard, D. A., Lin, R., Cerione, R. A. & Manor, D. Biochemical studies of the mechanism of action of the Cdc42-GTPase-activating protein. J. Biol. Chem. 273, 16210–16215 (1998).
Rittinger, K. et al. Crystal structure of a small G protein in complex with the GTPase-activating protein rhoGAP. Nature 388, 693–697 (1997).
Rittinger, K., Walker, P. A., Eccleston, J. F., Smerdon, S. J. & Gamblin, S. J. Structure at 1.65 A of RhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature 389, 758–762 (1997).
Hu, C. K., Coughlin, M., Field, C. M. & Mitchison, T. J. Cell polarization during monopolar cytokinesis. J. Cell Biol. 181, 195–202 (2008).
Toure, A. et al. Phosphoregulation of MgcRacGAP in mitosis involves Aurora B and Cdk1 protein kinases and the PP2A phosphatase. FEBS Lett. 582, 1182–8 (2008).
Fuller, B. G. et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 (2008).
Burkel, B. M., von Dassow, G. & Bement, W. M. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil. Cytoskeleton 64, 822–832 (2007).
Faire, K. et al. E-MAP-115 (ensconsin) associates dynamically with microtubules in vivo and is not a physiological modulator of microtubule dynamics. J. Cell Sci. 112, 4243–4255 (1999).
Kieserman, E. K., Glotzer, M. & Wallingford, J. B. Developmental regulation of central spindle assembly and cytokinesis during vertebrate embryogenesis. Curr. Biol. 18, 116–123 (2008).
Woolner, S., O'Brien, L. L., Wiese, C. & Bement, W. M. Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 182, 77–88 (2008).
Acknowledgements
We thank George von Dassow and Julie Canman for helpful discussions; Chloë Bulinski for the EMTB–3×GFP construct; Todd Stukenberg, Johné Liu and Aaron Straight for providing antibodies; John Wallingford and Esther Kieserman for advice on the midzone staining procedure; and members of the Bement Lab for advice and critical reading of the manuscript. A.L.M. is supported by postdoctoral fellowships from the American Cancer Society and the Helen Hay Whitney Foundation. W.M.B. is supported by an NIH grant.
Author information
Authors and Affiliations
Contributions
A.L.M. and W.M.B. conceived and designed the experiments; A.L.M. carried out the most of the experiments; W.M.B carried out the midzone staining experiments; A.L.M. wrote the manuscript and prepared the figures; W.M.B. provided feedback throughout the project and revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3113 kb)
Supplementary Information
Supplementary Movie 1 (MOV 2247 kb)
Supplementary Information
Supplementary Movie 2 (MOV 4853 kb)
Supplementary Information
Supplementary Movie 3 (MOV 2872 kb)
Supplementary Information
Supplementary Movie 4 (MOV 7848 kb)
Supplementary Information
Supplementary Movie 5 (MOV 2299 kb)
Supplementary Information
Supplementary Movie 6 (MOV 2637 kb)
Supplementary Information
Supplementary Movie 7 (MOV 5811 kb)
Rights and permissions
About this article
Cite this article
Miller, A., Bement, W. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol 11, 71–77 (2009). https://doi.org/10.1038/ncb1814
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1814
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
RacGAP1 promotes the malignant progression of cervical cancer by regulating AP-1 via miR-192 and p-JNK
Cell Death & Disease (2022)
-
Three-dimensional residual channel attention networks denoise and sharpen fluorescence microscopy image volumes
Nature Methods (2021)
-
The DNA-helicase HELLS drives ALK− ALCL proliferation by the transcriptional control of a cytokinesis-related program
Cell Death & Disease (2021)
-
Stress-mediated convergence of splicing landscapes in male and female rock doves
BMC Genomics (2020)