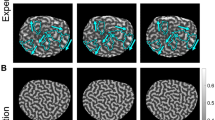
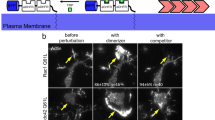
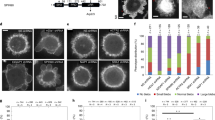
Abstract
The Rho GTPases — RHOA, RAC1 and CDC42 — are small GTP binding proteins that regulate basic biological processes such as cell locomotion, cell division and morphogenesis by promoting cytoskeleton-based changes in the cell cortex. This regulation results from active (GTP-bound) Rho GTPases stimulating target proteins that, in turn, promote actin assembly and myosin 2-based contraction to organize the cortex. This basic regulatory scheme, well supported by in vitro studies, led to the natural assumption that Rho GTPases function in vivo in an essentially linear matter, with a given process being initiated by GTPase activation and terminated by GTPase inactivation. However, a growing body of evidence based on live cell imaging, modelling and experimental manipulation indicates that Rho GTPase activation and inactivation are often tightly coupled in space and time via signalling circuits and networks based on positive and negative feedback. In this Review, we present and discuss this evidence, and we address one of the fundamental consequences of coupled activation and inactivation: the ability of the Rho GTPases to self-organize, that is, direct their own transition from states of low order to states of high order. We discuss how Rho GTPase self-organization results in the formation of diverse spatiotemporal cortical patterns such as static clusters, oscillatory pulses, travelling wave trains and ring-like waves. Finally, we discuss the advantages of Rho GTPase self-organization and pattern formation for cell function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
15 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41580-024-00701-7
References
Hodge, R. G. & Ridley, A. J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 17, 496–510 (2016).
Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Scheffzek, K. & Ahmadian, M. R. GTPase activating proteins: structural and functional insights 18 years after discovery. Cell Mol. Life Sci. 62, 3014–3038 (2005).
Rossman, K. L., Der, C. J. & Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 (2005).
Michaud, A. et al. Cortical excitability and cell division. Curr. Biol. 31, R553–R559 (2021).
Allen, W. E., Zicha, D., Ridley, A. J. & Jones, G. E. A role for Cdc42 in macrophage chemotaxis. J. Cell Biol. 141, 1147–1157 (1998).
Kishi, K., Sasaki, T., Kuroda, S., Itoh, T. & Takai, Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). J. Cell Biol. 120, 1187–1195 (1993).
Massol, P., Montcourrier, P., Guillemot, J. C. & Chavrier, P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 17, 6219–6229 (1998).
Stowers, L., Yelon, D., Berg, L. J. & Chant, J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc. Natl Acad. Sci. USA 92, 5027–5031 (1995).
Barrett, K., Leptin, M. & Settleman, J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905–915 (1997).
Howell, A. S. et al. Singularity in polarization: rewiring yeast cells to make two buds. Cell 139, 731–743 (2009).
Das, M. et al. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science 337, 239–243 (2012).
Michaux, J. B., Robin, F. B., McFadden, W. M. & Munro, E. M. Excitable RhoA dynamics drive pulsed contractions in the early C. elegans embryo. J. Cell Biol. 217, 4230–4252 (2018).
Abreu-Blanco, M. T., Verboon, J. M. & Parkhurst, S. M. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr. Biol. 24, 144–155 (2014).
Bement, W. M. et al. Activator-inhibitor coupling between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17, 1471–1483 (2015).
Bischof, J. et al. A cdk1 gradient guides surface contraction waves in oocytes. Nat. Commun. 8, 849 (2017).
Burkel, B. M., Benink, H. A., Vaughan, E. M., von Dassow, G. & Bement, W. M. A Rho GTPase signal treadmill backs a contractile array. Dev. Cell 23, 384–396 (2012).
Graessl, M. et al. An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J. Cell Biol. 216, 4271–4285 (2017).
Wu, M., Wu, X. & De Camilli, P. Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations. Proc. Natl Acad. Sci. USA 110, 1339–1344 (2013).
Xiao, S., Tong, C., Yang, Y. & Wu, M. Mitotic cortical waves predict future division sites by encoding positional and size information. Dev. Cell 43, 493–506.e3 (2017).
Landino, J. et al. Rho and F-actin self-organize within an artificial cell cortex. Curr. Biol. 31, 5613–5621.e5 (2021).
Ozbudak, E. M., Becskei, A. & van Oudenaarden, A. A system of counteracting feedback loops regulates Cdc42p activity during spontaneous cell polarization. Dev. Cell 9, 565–571 (2005).
Bement, W. M., Benink, H. A. & von Dassow, G. A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol. 170, 91–101 (2005).
Benink, H. A. & Bement, W. M. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–439 (2005).
Sokac, A. M., Co, C., Taunton, J. & Bement, W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 5, 727–732 (2003).
Nalbant, P., Hodgson, L., Kraynov, V., Toutchkine, A. & Hahn, K. M. Activation of endogenous Cdc42 visualized in living cells. Science 305, 1615–1619 (2004).
Machacek, M. et al. Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103 (2009).
Somers, W. G. & Saint, R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4, 29–39 (2003).
Bement, W. M., Miller, A. L. & von Dassow, G. Rho GTPase activity zones and transient contractile arrays. Bioessays 28, 983–993 (2006).
Goryachev, A. B. & Pokhilko, A. V. Computational model explains high activity and rapid cycling of Rho GTPases within protein complexes. PLoS Comput. Biol. 2, e172 (2006).
Clay, M. R. & Halloran, M. C. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development 140, 3198–3209 (2013).
Mason, F. M., Xie, S., Vasquez, C. G., Tworoger, M. & Martin, A. C. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J. Cell Biol. 214, 603–617 (2016).
Budnar, S. et al. Anillin promotes cell contractility by cyclic resetting of RhoA residence kinetics. Dev. Cell 49, 894–906.e12 (2019).
Bendezu, F. O. et al. Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking. PLoS Biol. 13, e1002097 (2015).
van Bruggen, R., Anthony, E., Fernandez-Borja, M. & Roos, D. Continuous translocation of Rac2 and the NADPH oxidase component p67phox during phagocytosis. J. Biol. Chem. 279, 9097–9102 (2004).
Goryachev, A. B. & Pokhilko, A. V. Dynamics of Cdc42 network embodies a turing-type mechanism of yeast cell polarity. FEBS Lett. 582, 1437–1443 (2008).
Tatebe, H., Nakano, K., Maximo, R. & Shiozaki, K. Pom1 DYRK regulates localization of the Rga4 GAP to ensure bipolar activation of Cdc42 in fission yeast. Curr. Biol. 18, 322–330 (2008).
Simões, S. et al. Compartmentalisation of Rho regulators directs cell invagination during tissue morphogenesis. Development 133, 4257–4267 (2006).
di Pietro, F. et al. Systematic analysis of RhoGEF/GAP localizations uncovers regulators of mechanosensing and junction formation during epithelial cell division. Curr. Biol. 33, 858–874.e7 (2023).
Müller, P. M. et al. Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat. Cell Biol. 22, 498–511 (2020).
Allard, J. & Mogilner, A. Traveling waves in actin dynamics and cell motility. Curr. Opin. Cell Biol. 25, 107–115 (2013).
Goryachev, A. B., Leda, M., Miller, A. L., von Dassow, G. & Bement, W. M. How to make a static cytokinetic furrow out of traveling excitable waves. Small GTPases 7, 65–70 (2016).
Landge, A. N., Jordan, B. M., Diego, X. & Müller, P. Pattern formation mechanisms of self-organizing reaction-diffusion systems. Dev. Biol. 460, 2–11 (2020).
Mitchison, T. J. & Field, C. M. Self-organization of cellular units. Annu. Rev. Cell Dev. Biol. 37, 23–41 (2021).
Valdez, V. A., Neahring, L., Petry, S. & Dumont, S. Mechanisms underlying spindle assembly and robustness. Nat. Rev. Mol. Cell Biol. 24, 523–542 (2023).
Heald, R. et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996).
Collinet, C. & Lecuit, T. Programmed and self-organized flow of information during morphogenesis. Nat. Rev. Mol. Cell Biol. 22, 245–265 (2021).
Hansen, S. D. et al. Stochastic geometry sensing and polarization in a lipid kinase–phosphatase competitive reaction. Proc. Natl Acad. Sci. USA 116, 15013–15022 (2019).
Murray, J. D. Mathematical Biology 19 (Springer-Verlag, 1993).
Meinhardt, H. & Gierer, A. Pattern formation by local self-activation and lateral inhibition. Bioessays 22, 753–760 (2000).
Rao, C. V., Wolf, D. M. & Arkin, A. P. Control, exploitation and tolerance of intracellular noise. Nature 420, 231–237 (2002).
Chiou, J.-g, Moran, K. D. & Lew, D. J. How cells determine the number of polarity sites. Preprint at bioRxiv https://doi.org/10.1101/2020.05.21.109520 (2020).
Hennig, K. et al. Stick–slip dynamics of cell adhesion triggers spontaneous symmetry breaking and directional migration of mesenchymal cells on one-dimensional lines. Sci. Adv. 6, eaau5670 (2020).
Lavrsen, K. et al. Microtubule detyrosination drives symmetry breaking to polarize cells for directed cell migration. Proc. Natl Acad. Sci. USA 120, e2300322120 (2023).
Hoyle, R. Pattern Formation (Cambridge Univ. Press, 2006).
Cross, M. C. & Greenside, H. Pattern Formation and Dynamics in Nonequilibrium Systems (Cambridge Univ. Press, 2009).
Miller, A. L. & Bement, W. M. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 11, 71–77 (2009).
Zheng, Y., Bender, A. & Cerione, R. A. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J. Biol. Chem. 270, 626–630 (1995).
Loose, M., Fischer-Friedrich, E., Ries, J., Kruse, K. & Schwille, P. Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science 320, 789–792 (2008).
Ramm, B., Glock, P. & Schwille, P. In vitro reconstitution of self-organizing protein patterns on supported lipid bilayers. J. Vis. Exp. 28, 58139 (2018).
Cezanne, A., Lauer, J., Solomatina, A., Sbalzarini, I. F. & Zerial, M. A non-linear system patterns Rab5 GTPase on the membrane. eLife 9, e54434 (2020).
Goryachev, A. B. & Leda, M. Autoactivation of small GTPases by the GEF-effector positive feedback modules. F1000Res 8, https://doi.org/10.12688/f1000research.20003.1 (2019).
Jackson, C. L. GEF–effector interactions. Cell Logist. 4, e943616 (2014).
Horiuchi, H. et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149–1159 (1997).
Grosshans, B. L., Ortiz, D. & Novick, P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl Acad. Sci. USA 103, 11821–11827 (2006).
Chen, Z. et al. Activated RhoA binds to the pleckstrin homology (PH) domain of PDZ-RhoGEF, a potential site for autoregulation. J. Biol. Chem. 285, 21070–21081 (2010).
Medina, F. et al. Activated RhoA is a positive feedback regulator of the Lbc family of Rho guanine nucleotide exchange factor proteins. J. Biol. Chem. 288, 11325–11333 (2013).
Chen, M. et al. Structure and regulation of human epithelial cell transforming 2 protein. Proc. Natl Acad. Sci. USA 117, 1027–1035 (2020).
Lin, Q., Yang, W., Baird, D., Feng, Q. & Cerione, R. A. Identification of a DOCK180-related guanine nucleotide exchange factor that is capable of mediating a positive feedback activation of Cdc42. J. Biol. Chem. 281, 35253–35262 (2006).
Bose, I. et al. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276, 7176–7186 (2001).
Butty, A. C. et al. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 21, 1565–1576 (2002).
Hussain, N. K. et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat. Cell Biol. 3, 927–932 (2001).
Lamas, I., Weber, N. & Martin, S. G. Activation of Cdc42 GTPase upon CRY2-induced cortical recruitment is antagonized by GAPs in fission yeast. Cells 9, 2089 (2020).
McGavin, M. K. et al. The intersectin 2 adaptor links Wiskott Aldrich syndrome protein (WASp)-mediated actin polymerization to T cell antigen receptor endocytosis. J. Exp. Med. 194, 1777–1787 (2001).
Castro-Castro, A. et al. Coronin 1A promotes a cytoskeletal-based feedback loop that facilitates Rac1 translocation and activation. EMBO J. 30, 3913–3927 (2011).
Segal, D., Zaritsky, A., Schejter, E. D. & Shilo, B. Z. Feedback inhibition of actin on Rho mediates content release from large secretory vesicles. J. Cell Biol. 217, 1815–1826 (2018).
Nguyen, T. T. et al. PLEKHG3 enhances polarized cell migration by activating actin filaments at the cell front. Proc. Natl Acad. Sci. USA 113, 10091–10096 (2016).
DerMardirossian, C., Schnelzer, A. & Bokoch, G. M. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell 15, 117–127 (2004).
Kitzing, T. M. et al. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes. Dev. 21, 1478–1483 (2007).
Priya, R. et al. Feedback regulation through myosin II confers robustness on RhoA signalling at E-cadherin junctions. Nat. Cell Biol. 17, 1282–1293 (2015).
Barrows, D. et al. p21-activated kinases (PAKs) mediate the phosphorylation of PREX2 protein to initiate feedback inhibition of Rac1 GTPase. J. Biol. Chem. 290, 28915–28931 (2015).
Radu, M. et al. ArhGAP15, a Rac-specific GTPase-activating protein, plays a dual role in inhibiting small GTPase signaling. J. Biol. Chem. 288, 21117–21125 (2013).
Dibus, M., Brábek, J. & Rösel, D. A screen for PKN3 substrates reveals an activating phosphorylation of ARHGAP18. Int J. Mol. Sci. 21, 7769 (2020).
Diring, J., Mouilleron, S., McDonald, N. Q. & Treisman, R. RPEL-family rhoGAPs link Rac/Cdc42 GTP loading to G-actin availability. Nat. Cell Biol. 21, 845–855 (2019).
Lee, C. S., Choi, C. K., Shin, E. Y., Schwartz, M. A. & Kim, E. G. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J. Cell Biol. 190, 663–674 (2010).
Premkumar, L. et al. Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs). J. Biol. Chem. 285, 13211–13222 (2010).
Kunisaki, Y. et al. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J. Cell Biol. 174, 647–652 (2006).
Kanai, A. et al. Identification of DOCK4 and its splicing variant as PIP3 binding proteins. IUBMB Life 60, 467–472 (2008).
Fritsch, R. et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 153, 1050–1063 (2013).
Zheng, Y., Bagrodia, S. & Cerione, R. A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 269, 18727–18730 (1994).
Goryachev, A. B. & Leda, M. Many roads to symmetry breaking: molecular mechanisms and theoretical models of yeast cell polarity. Mol. Biol. Cell 28, 370–380 (2017).
Cherfils, J. & Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 93, 269–309 (2013).
Hennig, A., Markwart, R., Esparza-Franco, M. A., Ladds, G. & Rubio, I. Ras activation revisited: role of GEF and GAP systems. Biol. Chem. 396, 831–848 (2015).
Arkowitz, R. A. & Bassilana, M. Polarized growth in fungi: symmetry breaking and hyphal formation. Semin. Cell Dev. Biol. 22, 806–815 (2011).
Martin, S. G. & Arkowitz, R. A. Cell polarization in budding and fission yeasts. FEMS Microbiol. Rev. 38, 228–253 (2014).
Bi, E. & Park, H. O. Cell polarization and cytokinesis in budding yeast. Genetics 191, 347–387 (2012).
Chiou, J. G., Balasubramanian, M. K. & Lew, D. J. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 33, 77–101 (2017).
Richman, T. J., Sawyer, M. M. & Johnson, D. I. Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1, 458–468 (2002).
Adams, A. E., Johnson, D. I., Longnecker, R. M., Sloat, B. F. & Pringle, J. R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111, 131–142 (1990).
Johnson, D. I. & Pringle, J. R. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111, 143–152 (1990).
Wedlich-Soldner, R., Wai, S. C., Schmidt, T. & Li, R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166, 889–900 (2004).
Tong, Z. et al. Adjacent positioning of cellular structures enabled by a Cdc42 GTPase-activating protein-mediated zone of inhibition. J. Cell Biol. 179, 1375–1384 (2007).
Okada, S., Lee, M. E., Bi, E. & Park, H. O. Probing Cdc42 polarization dynamics in budding yeast using a biosensor. Methods Enzymol. 589, 171–190 (2017).
Lichius, A. et al. CDC-42 and RAC-1 regulate opposite chemotropisms in Neurospora crassa. J. Cell Sci. 127, 1953–1965 (2014).
Okada, S. et al. Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev. Cell 26, 148–161 (2013).
Caudron, F. & Barral, Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16, 493–506 (2009).
Virag, A., Lee, M. P., Si, H. & Harris, S. D. Regulation of hyphal morphogenesis by Cdc42 and Rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 66, 1579–1596 (2007).
Böhmer, C., Ripp, C. & Bölker, M. The germinal centre kinase Don3 triggers the dynamic rearrangement of higher-order septin structures during cytokinesis in Ustilago maydis. Mol. Microbiol. 74, 1484–1496 (2009).
Kayano, Y., Tanaka, A. & Takemoto, D. Two closely related Rho GTPases, Cdc42 and RacA, of the en-dophytic fungus Epichloë festucae have contrasting roles for ROS production and symbiotic infection synchronized with the host plant. PLoS Pathog. 14, e1006840 (2018).
Pan, X., Pérez-Henríquez, P., Van Norman, J. M. & Yang, Z. Membrane nanodomains: dynamic nanobuilding blocks of polarized cell growth. Plant. Physiol. 193, 83–97 (2023).
Li, E. et al. Signaling network controlling ROP-mediated tip growth in Arabidopsis and beyond. Plant. Commun. 4, 100451 (2023).
Ou, H. & Yi, P. ROP GTPase-dependent polarity establishment during tip growth in plants. N. phytol. 236, 49–57 (2022).
Mulvey, H. & Dolan, L. RHO GTPase of plants regulates polarized cell growth and cell division orientation during morphogenesis. Curr. Biol. 33, 2897–2911.e6 (2023).
Drubin, D. G. Development of cell polarity in budding yeast. Cell 65, 1093–1096 (1991).
Park, H. O. & Bi, E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71, 48–96 (2007).
Ayscough, K. R. et al. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399–416 (1997).
Butty, A. C., Pryciak, P. M., Huang, L. S., Herskowitz, I. & Peter, M. The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282, 1511–1516 (1998).
Li, R. & Bowerman, B. Symmetry breaking in biology. Cold Spring Harb. Perspect. Biol. 2, a003475 (2010).
Irazoqui, J. E., Gladfelter, A. S. & Lew, D. J. Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5, 1062–1070 (2003).
Kozubowski, L. et al. Symmetry-breaking polarization driven by a Cdc42p GEF–PAK complex. Curr. Biol. 18, 1719–1726 (2008).
Woods, B., Kuo, C. C., Wu, C. F., Zyla, T. R. & Lew, D. J. Polarity establishment requires localized activation of Cdc42. J. Cell Biol. 211, 19–26 (2015).
Witte, K., Strickland, D. & Glotzer, M. Cell cycle entry triggers a switch between two modes of Cdc42 activation during yeast polarization. eLife 6, e26722 (2017).
Chang, E. C. et al. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79, 131–141 (1994).
Lamas, I., Merlini, L., Vještica, A., Vincenzetti, V. & Martin, S. G. Optogenetics reveals Cdc42 local activation by scaffold-mediated positive feedback and Ras GTPase. PLoS Biol. 18, e3000600 (2020).
Altschuler, S. J., Angenent, S. B., Wang, Y. & Wu, L. F. On the spontaneous emergence of cell polarity. Nature 454, 886–889 (2008).
Wedlich-Soldner, R., Altschuler, S., Wu, L. & Li, R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231–1235 (2003).
Wu, C. F., Savage, N. S. & Lew, D. J. Interaction between bud-site selection and polarity-establishment machineries in budding yeast. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130006 (2013).
Wu, C. F. et al. Role of competition between polarity sites in establishing a unique front. eLife 4, e11611 (2015).
Otsuji, M. et al. A mass conserved reaction-diffusion system captures properties of cell polarity. PLoS Comput. Biol. 3, e108 (2007).
Chiou, J. G. et al. Principles that govern competition or co-existence in Rho-GTPase driven polarization. PLoS Comput. Biol. 14, e1006095 (2018).
Brauns, F., Halatek, J. & Frey, E. Phase-space geometry of mass-conserving reaction-diffusion dynamics. Phys. Rev. X 10, 041036 (2020).
Verschueren, N. & Champneys, A. A model for cell polarization without mass conservation. SIAM J. Appl. Dyn. Syst. 16, 1797–1830 (2017).
Goryachev, A. B. & Leda, M. Compete or coexist? Why the same mechanisms of symmetry breaking can yield distinct outcomes. Cells 9, 2011 (2020).
Mori, Y., Jilkine, A. & Edelstein-Keshet, L. Wave-pinning and cell polarity from a bistable reaction-diffusion system. Biophys. J. 94, 3684–3697 (2008).
Smith, S. E. et al. Independence of symmetry breaking on Bem1-mediated autocatalytic activation of Cdc42. J. Cell Biol. 202, 1091–1106 (2013).
Klunder, B., Freisinger, T., Wedlich-Soldner, R. & Frey, E. GDI-mediated cell polarization in yeast provides precise spatial and temporal control of Cdc42 signaling. PLoS Comput. Biol. 9, e1003396 (2013).
Freisinger, T. et al. Establishment of a robust single axis of cell polarity by coupling multiple positive feedback loops. Nat. Commun. 4, 1807 (2013).
Jose, M., Tollis, S., Nair, D., Sibarita, J. B. & McCusker, D. Robust polarity establishment occurs via an endocytosis-based cortical corralling mechanism. J. Cell Biol. 200, 407–418 (2013).
Giese, W., Eigel, M., Westerheide, S., Engwer, C. & Klipp, E. Influence of cell shape, inhomogeneities and diffusion barriers in cell polarization models. Phys. Biol. 12, 066014 (2015).
Lee, M. E., Lo, W. C., Miller, K. E., Chou, C. S. & Park, H. O. Regulation of Cdc42 polarization by the Rsr1 GTPase and Rga1, a Cdc42 GTPase-activating protein, in budding yeast. J. Cell Sci. 128, 2106–2117 (2015).
Lo, W. C., Lee, M. E., Narayan, M., Chou, C. S. & Park, H. O. Polarization of diploid daughter cells directed by spatial cues and GTP hydrolysis of Cdc42 budding yeast. PLoS ONE 8, e56665 (2013).
Bonazzi, D. et al. Actin-based transport adapts polarity domain size to local cellular curvature. Curr. Biol. 25, 2677–2683 (2015).
Bonazzi, D. et al. Symmetry breaking in spore germination relies on an interplay between polar cap stability and spore wall mechanics. Dev. Cell 28, 534–546 (2014).
Daalman, W. K., Sweep, E. & Laan, L. A tractable physical model for the yeast polarity predicts epistasis and fitness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220044 (2023).
Kuo, C. C. et al. Inhibitory GEF phosphorylation provides negative feedback in the yeast polarity circuit. Curr. Biol. 24, 753–759 (2014).
Dyer, J. M. et al. Tracking shallow chemical gradients by actin-driven wandering of the polarization site. Curr. Biol. 23, 32–41 (2013).
Howell, A. S. et al. Negative feedback enhances robustness in the yeast polarity establishment circuit. Cell 149, 322–333 (2012).
Khalili, B., Merlini, L., Vincenzetti, V., Martin, S. G. & Vavylonis, D. Exploration and stabilization of Ras1 mating zone: a mechanism with positive and negative feedbacks. PLoS Comput. Biol. 14, e1006317 (2018).
Ghose, D. & Lew, D. Mechanistic insights into actin-driven polarity site movement in yeast. Mol. Biol. Cell 31, 1085–1102 (2020).
Layton, A. T. et al. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr. Biol. 21, 184–194 (2011).
Watson, L. J., Rossi, G. & Brennwald, P. Quantitative analysis of membrane trafficking in regulation of Cdc42 polarity. Traffic 15, 1330–1343 (2014).
Gerganova, V. et al. Cell patterning by secretion-induced plasma membrane flows. Sci. Adv. 7, eabg6718 (2021).
Khalili, B., Lovelace, H. D., Rutkowski, D. M., Holz, D. & Vavylonis, D. Fission yeast polarization: modeling Cdc42 oscillations, symmetry breaking, and zones of activation and inhibition. Cells 9, 1769 (2020).
Pino, M. R. et al. Cdc42 GTPase-activating proteins (GAPs) regulate generational inheritance of cell polarity and cell shape in fission yeast. Mol. Biol. Cell 32, ar14 (2021).
Coravos, J. S., Mason, F. M. & Martin, A. C. Actomyosin pulsing in tissue integrity maintenance during morphogenesis. Trends Cell Biol. 27, 276–283 (2017).
Munjal, A., Philippe, J. M., Munro, E. & Lecuit, T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355 (2015).
Munjal, A. & Lecuit, T. Actomyosin networks and tissue morphogenesis. Development 141, 1789–1793 (2014).
Coravos, J. S. & Martin, A. C. Apical sarcomere-like actomyosin contracts nonmuscle Drosophila epithelial cells. Dev. Cell 39, 346–358 (2016).
Kim, H. Y. & Davidson, L. A. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J. Cell Sci. 124, 635–646 (2011).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Costache, V. et al. Rapid assembly of a polar network architecture drives efficient actomyosin contractility. Cell Rep. 39, 110868 (2022).
Mason, F. M., Tworoger, M. & Martin, A. C. Apical domain polarization localizes actin–myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 15, 926–936 (2013).
Nishikawa, M., Naganathan, S. R., Jülicher, F. & Grill, S. W. Controlling contractile instabilities in the actomyosin cortex. eLife 6, e19595 (2017).
Schmutz, C., Stevens, J. & Spang, A. Functions of the novel RhoGAP proteins RGA-3 and RGA-4 in the germ line and in the early embryo of C. elegans. Development 134, 3495–3505 (2007).
Baird, M. A. et al. Local pulsatile contractions are an intrinsic property of the myosin 2A motor in the cortical cytoskeleton of adherent cells. Mol. Biol. Cell 28, 240–251 (2017).
Beach, J. R. et al. Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nat. Cell Biol. 19, 85–93 (2017).
Lehtimäki, J. I., Rajakylä, E. K., Tojkander, S. & Lappalainen, P. Generation of stress fibers through myosin-driven reorganization of the actin cortex. eLife 10, e60710 (2021).
Kumari, A. et al. Actomyosin-driven force patterning controls endocytosis at the immune synapse. Nat. Commun. 10, 2870 (2019).
Kamps, D. et al. Optogenetic tuning reveals Rho amplification-dependent dynamics of a cell contraction signal network. Cell Rep. 33, 108467 (2020).
Chen, X., Venkatachalapathy, M., Dehmelt, L. & Wu, Y. W. Multidirectional activity control of cellular processes by a versatile chemo-optogenetic approach. Angew. Chem. Int. Ed. Engl. 57, 11993–11997 (2018).
Beta, C., Edelstein-Keshet, L., Gov, N. & Yochelis, A. From actin waves to mechanism and back: how theory aids biological understanding. eLife 12, e87181 (2023).
Sokac, A. M. & Bement, W. M. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol. Biol. Cell 17, 1495–1502 (2006).
Nemoto, T., Kojima, T., Oshima, A., Bito, H. & Kasai, H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J. Biol. Chem. 279, 37544–37550 (2004).
Rousso, T., Schejter, E. D. & Shilo, B. Z. Orchestrated content release from Drosophila glue-protein vesicles by a contractile actomyosin network. Nat. Cell Biol. 18, 181–190 (2016).
Ma, W. et al. Arp2/3 nucleates F-actin coating of fusing insulin granules in pancreatic β cells to control insulin secretion. J. Cell Sci. 133, jcs236794 (2020).
Miklavc, P., Wittekindt, O. H., Felder, E. & Dietl, P. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: a prerequisite for content release or kiss-and-run. Ann. N. Y. Acad. Sci. 1152, 43–52 (2009).
Nightingale, T. D. et al. Actomyosin II contractility expels von Willebrand factor from Weibel–Palade bodies during exocytosis. J. Cell Biol. 194, 613–629 (2011).
Yu, H. Y. & Bement, W. M. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat. Cell Biol. 9, 149–159 (2007).
Yu, H. Y. & Bement, W. M. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol. Biol. Cell 18, 4096–4105 (2007).
Miklavc, P. et al. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion—a role for Rho, formins and myosin II. J. Cell Sci. 125, 2765–2774 (2012).
Tran, D. T., Masedunskas, A., Weigert, R. & Ten Hagen, K. G. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat. Commun. 6, 10098 (2015).
Mietkowska, M., Schuberth, C., Wedlich-Söldner, R. & Gerke, V. Actin dynamics during Ca2+-dependent exocytosis of endothelial Weibel–Palade bodies. Biochim. Biophys. Acta Mol. Cell Res. 1866, 1218–1229 (2019).
Sokac, A. M., Schietroma, C., Gundersen, C. B. & Bement, W. M. Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 11, 629–640 (2006).
Kamalesh, K. et al. Exocytosis by vesicle crumpling maintains apical membrane homeostasis during exocrine secretion. Dev. Cell 56, 1603–1616.e6 (2021).
Miklavc, P. et al. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 128, 1193–1203 (2015).
Kono, K., Saeki, Y., Yoshida, S., Tanaka, K. & Pellman, D. Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell 150, 151–164 (2012).
Xu, J. et al. Redox-sensitive CDC-42 clustering promotes wound closure in C. elegans. Cell Rep. 37, 110040 (2021).
Horn, A. et al. Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci. Signal. 10, eaaj1978 (2017).
Moe, A. M., Golding, A. E. & Bement, W. M. Cell healing: calcium, repair and regeneration. Semin. Cell Dev. Biol. 45, 18–23 (2015).
Verboon, J. M. & Parkhurst, S. M. Rho family GTPases bring a familiar ring to cell wound repair. Small GTPases 6, 1–7 (2015).
Golding, A. E., Visco, I., Bieling, P. & Bement, W. M. Extraction of active RhoGTPases by RhoGDI regulates spatiotemporal patterning of RhoGTPases. eLife 8, e50471 (2019).
Vaughan, E. M., Miller, A. L., Yu, H. Y. & Bement, W. M. Control of local Rho GTPase crosstalk by Abr. Curr. Biol. 21, 270–277 (2011).
Nakamura, M., Verboon, J. M. & Parkhurst, S. M. Prepatterning by RhoGEFs governs Rho GTPase spatiotemporal dynamics during wound repair. J. Cell Biol. 216, 3959–3969 (2017).
Mandato, C. A. & Bement, W. M. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 154, 785–797 (2001).
Hui, J., Nakamura, M., Dubrulle, J. & Parkhurst, S. M. Coordinated efforts of different actin filament populations are needed for optimal cell wound repair. Mol. Biol. Cell 34, ar15 (2023).
Abreu-Blanco, M. T., Verboon, J. M. & Parkhurst, S. M. Cell wound repair in Drosophila occurs through three distinct phases of membrane and cytoskeletal remodeling. J. Cell Biol. 193, 455–464 (2011).
Holmes, W. R., Golding, A. E., Bement, W. M. & Edelstein-Keshet, L. A mathematical model of GTPase pattern formation during single-cell wound repair. Interface Focus. 6, 20160032 (2016).
Simon, C. M., Vaughan, E. M., Bement, W. M. & Edelstein-Keshet, L. Pattern formation of Rho GTPases in single cell wound healing. Mol. Biol. Cell 24, 421–432 (2013).
Chuang, T. H. et al. Abr and Bcr are multifunctional regulators of the Rho GTP-binding protein family. Proc. Natl Acad. Sci. USA 92, 10282–10286 (1995).
Davenport, N. R., Sonnemann, K. J., Eliceiri, K. W. & Bement, W. M. Membrane dynamics during cellular wound repair. Mol. Biol. Cell 27, 2272–2285 (2016).
Vaughan, E. M. et al. Lipid domain-dependent regulation of single-cell wound repair. Mol. Biol. Cell 25, 1867–1876 (2014).
Moe, A. et al. Cross-talk-dependent cortical patterning of Rho GTPases during cell repair. Mol. Biol. Cell 32, 1417–1432 (2021).
Yüce, O., Piekny, A. & Glotzer, M. An ECT2–centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571–582 (2005).
Wagner, E. & Glotzer, M. Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J. Cell Biol. 213, 641–649 (2016).
Bastos, R. N., Penate, X., Bates, M., Hammond, D. & Barr, F. A. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. J. Cell Biol. 198, 865–880 (2012).
Su, K. C., Takaki, T. & Petronczki, M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev. Cell 21, 1104–1115 (2011).
Nishimura, Y. & Yonemura, S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J. Cell Sci. 119, 104–114 (2006).
Jordan, S. N. & Canman, J. C. Rho GTPases in animal cell cytokinesis: an occupation by the one percent. Cytoskeleton 69, 919–930 (2012).
Zhuravlev, Y. et al. CYK-4 regulates Rac, but not Rho, during cytokinesis. Mol. Biol. Cell 28, 1258–1270 (2017).
Canman, J. C. et al. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322, 1543–1546 (2008).
Zhang, D. & Glotzer, M. The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. eLife 4, e08898 (2015).
Loria, A., Longhini, K. M. & Glotzer, M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr. Biol. 22, 213–219 (2012).
Swider, Z. T. et al. Cell cycle and developmental control of cortical excitability in Xenopus laevis. Mol. Biol. Cell 33, ar73 (2022).
Michaud, A. et al. A versatile cortical pattern-forming circuit based on Rho, F-actin, Ect2, and RGA-3/4. J. Cell Biol. 221, e202203017 (2022).
Zanin, E. et al. A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev. Cell 26, 496–510 (2013).
Bell, K. R. et al. Novel cytokinetic ring components drive negative feedback in cortical contractility. Mol. Biol. Cell 31, 1623–1636 (2020).
Krendel, M., Zenke, F. T. & Bokoch, G. M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat. Cell Biol. 4, 294–301 (2002).
Tong, C. S., Xu, X. J. & Wu, M. Periodicity, mixed-mode oscillations, and multiple timescale in a phosphoinositide–Rho GTPase network. Cell Rep. 42, 112857 (2023).
Scott, D. W., Tolbert, C. E. & Burridge, K. Tension on JAM-A activates RhoA via GEF-H1 and p115 RhoGEF. Mol. Biol. Cell 27, 1420–1430 (2016).
Scholze, M. J. et al. PI(4,5)P(2) forms dynamic cortical structures and directs actin distribution as well as polarity in Caenorhabditis elegans embryos. Development 145, dev164988 (2018).
Reyes, C. C. et al. Anillin regulates cell–cell junction integrity by organizing junctional accumulation of Rho-GTP and actomyosin. Curr. Biol. 24, 1263–1270 (2014).
Terry, S. J. et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat. Cell Biol. 13, 159–166 (2011).
Ratheesh, A., Priya, R. & Yap, A. S. Coordinating Rho and Rac: the regulation of Rho GTPase signaling and cadherin junctions. Prog. Mol. Biol. Transl. Sci. 116, 49–68 (2013).
Varadarajan, S., Stephenson, R. E. & Miller, A. L. Multiscale dynamics of tight junction remodeling. J. Cell Sci. 132, jcs229286 (2019).
Citi, S., Guerrera, D., Spadaro, D. & Shah, J. Epithelial junctions and Rho family GTPases: the zonular signalosome. Small GTPases 5, 1–15 (2014).
Arnold, T. R., Stephenson, R. E. & Miller, A. L. Rho GTPases and actomyosin: partners in regulating epithelial cell–cell junction structure and function. Exp. Cell Res. 358, 20–30 (2017).
Ratheesh, A. et al. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat. Cell Biol. 14, 818–828 (2012).
Breznau, E. B., Semack, A. C., Higashi, T. & Miller, A. L. MgcRacGAP restricts active RhoA at the cytokinetic furrow and both RhoA and Rac1 at cell–cell junctions in epithelial cells. Mol. Biol. Cell 26, 2439–2455 (2015).
Arnold, T. R. et al. Anillin regulates epithelial cell mechanics by structuring the medial–apical actomyosin network. eLife 8, e39062 (2019).
Priya, R. et al. Bistable front dynamics in a contractile medium: travelling wave fronts and cortical advection define stable zones of RhoA signaling at epithelial adherens junctions. PLoS Comput. Biol. 13, e1005411 (2017).
Stephenson, R. E. et al. Rho flares repair local tight junction leaks. Dev. Cell 48, 445–459.e5 (2019).
Varadarajan, S. et al. Mechanosensitive calcium flashes promote sustained RhoA activation during tight junction remodeling. J. Cell Biol. 221, e202102107 (2022).
Chumki, S. A., van den Goor, L. M., Hall, B. N. & Miller, A. L. p115RhoGEF activates RhoA to support tight junction maintenance and remodeling. Mol. Biol. Cell 33, ar136 (2022).
Weiner, O. D., Marganski, W. A., Wu, L. F., Altschuler, S. J. & Kirschner, M. W. An actin-based wave generator organizes cell motility. PLoS Biol. 5, e221 (2007).
Sonnemann, K. J. & Bement, W. M. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 27, 237–263 (2011).
Yao, B., Donoughe, S., Michaux, J. & Munro, E. Modulating RhoA effectors induces transitions to oscillatory and more wavelike RhoA dynamics in Caenorhabditis elegans zygotes. Mol. Biol. Cell 33, ar58 (2022).
Xie, S. & Martin, A. C. Intracellular signalling and intercellular coupling coordinate heterogeneous contractile events to facilitate tissue folding. Nat. Commun. 6, 7161 (2015).
Zulueta-Coarasa, T. & Fernandez-Gonzalez, R. Dynamic force patterns promote collective cell movements during embryonic wound repair. Nat. Phys. 14, 750–758 (2018).
Cavanaugh, K. E., Chmiel, T. A. & Gardel, M. L. Caveolae spelunking: exploring a new modality in tensional homeostasis. Dev. Cell 54, 3–5 (2020).
Mak, M., Zaman, M. H., Kamm, R. D. & Kim, T. Interplay of active processes modulates tension and drives phase transition in self-renewing, motor-driven cytoskeletal networks. Nat. Commun. 7, 10323 (2016).
Su, K. C., Bement, W. M., Petronczki, M. & von Dassow, G. An astral simulacrum of the central spindle accounts for normal, spindle-less, and anucleate cytokinesis in echinoderm embryos. Mol. Biol. Cell 25, 4049–4062 (2014).
Poirier, M. B., Fiorino, C., Rajasekar, T. K. & Harrison, R. E. F-Actin flashes on phagosomes mechanically deform contents for efficient digestion in macrophages. J. Cell Sci. 133, jcs239384 (2020).
Herron, J. C. et al. Spatial models of pattern formation during phagocytosis. PLoS Comput. Biol. 18, e1010092 (2022).
Kreider-Letterman, G. et al. ARHGAP17 regulates the spatiotemporal activity of Cdc42 at invadopodia. J. Cell Biol. 222, e202207020 (2023).
Reinhard, N. R. et al. Spatiotemporal analysis of RhoA/B/C activation in primary human endothelial cells. Sci. Rep. 6, 25502 (2016).
Case, L. B. & Waterman, C. M. Adhesive F-actin waves: a novel integrin-mediated adhesion complex coupled to ventral actin polymerization. PLoS ONE 6, e26631 (2011).
van Loon, A. P., Erofeev, I. S., Goryachev, A. B. & Sagasti, A. Stochastic contraction of myosin minifilaments drives evolution of microridge protrusion patterns in epithelial cells. Mol. Biol. Cell 32, 1501–1513 (2021).
van Loon, A. P., Erofeev, I. S., Maryshev, I. V., Goryachev, A. B. & Sagasti, A. Cortical contraction drives the 3D patterning of epithelial cell surfaces. J. Cell Biol. 219, e201904144 (2020).
Sternberg, H. et al. Formation of self-organizing functionally distinct Rho of plants domains involves a reduced mobile population. Plant. Physiol. 187, 2485–2508 (2021).
Fritz, R. D. & Pertz, O. The dynamics of spatio-temporal Rho GTPase signaling: formation of signaling patterns. F1000Res 5, https://doi.org/10.12688/f1000research.7370.1 (2016).
Bolado-Carrancio, A. et al. Periodic propagating waves coordinate RhoGTPase network dynamics at the leading and trailing edges during cell migration. eLife 9, e58165 (2020).
Yang, H. W., Collins, S. R. & Meyer, T. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat. Cell Biol. 18, 191–201 (2016).
Martin, K. et al. Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics. Sci. Rep. 6, 21901 (2016).
Huang, C. H., Tang, M., Shi, C., Iglesias, P. A. & Devreotes, P. N. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat. Cell Biol. 15, 1307–1316 (2013).
Mahlandt, E. K., Kreider-Letterman, G., Chertkova, A. O., Garcia-Mata, R. & Goedhart, J. Cell-based optimization and characterization of genetically encoded location-based biosensors for Cdc42 or Rac activity. J. Cell Sci. 136, jcs260802 (2023).
Mahlandt, E. K. et al. Visualizing endogenous Rho activity with an improved localization-based, genetically encoded biosensor. J. Cell Sci. 134, jcs258823 (2021).
Azoitei, M. L. et al. Spatiotemporal dynamics of GEF-H1 activation controlled by microtubule- and Src-mediated pathways. J. Cell Biol. 218, 3077–3097 (2019).
Ichikawa, T., Stuckenholz, C. & Davidson, L. A. Non-junctional role of Cadherin3 in cell migration and contact inhibition of locomotion via domain-dependent, opposing regulation of Rac1. Sci. Rep. 10, 17326 (2020).
Floerchinger, A. et al. Optimizing metastatic-cascade-dependent Rac1 targeting in breast cancer: guidance using optical window intravital FRET imaging. Cell Rep. 36, 109689 (2021).
Gupta, S. et al. Enhanced RhoA signalling stabilizes E-cadherin in migrating epithelial monolayers. J. Cell Sci. 134, jcs258767 (2021).
Nanda, S. et al. Rho GTPase activity crosstalk mediated by Arhgef11 and Arhgef12 coordinates cell protrusion-retraction cycles. Nat. Commun. 14, 8356 (2023).
Patwardhan, R. et al. Cdc42 activity in the trailing edge is required for persistent directional migration of keratinocytes. Mol Biol Cell. https://doi.org/10.1091/mbc.E23-08-0318 (2024).
Dada, O., Gutowski, S., Brautigam, C. A., Chen, Z. & Sternweis, P. C. Direct regulation of p190RhoGEF by activated Rho and Rac GTPases. J. Struct. Biol. 202, 13–24 (2018).
Chen, Z. et al. Activation of p115-RhoGEF requires direct association of Gα13 and the Dbl homology domain. J. Biol. Chem. 287, 25490–25500 (2012).
Baird, D., Feng, Q. & Cerione, R. A. The Cool-2/α-Pix protein mediates a Cdc42–Rac signaling cascade. Curr. Biol. 15, 1–10 (2005).
Bellanger, J. M. et al. The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin. Nat. Cell Biol. 2, 888–892 (2000).
Kim, K., Hou, P., Gorski, J. L. & Cooper, J. A. Effect of Fgd1 on cortactin in Arp2/3 complex-mediated actin assembly. Biochemistry 43, 2422–2427 (2004).
Nayal, A. et al. Paxillin phosphorylation at Ser273 localizes a GIT1–PIX–PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 173, 587–589 (2006).
Ten Klooster, J. P. et al. Interaction between Tiam1 and the Arp2/3 complex links activation of Rac to actin polymerization. Biochem. J. 397, 39–45 (2006).
Lee, C. C., Huang, C. C. & Hsu, K. S. The phospholipid-binding protein SESTD1 negatively regulates dendritic spine density by interfering with Rac1–Trio8 signaling pathway. Sci. Rep. 5, 13250 (2015).
Barrows, D., He, J. Z. & Parsons, R. PREX1 protein function is negatively regulated downstream of receptor tyrosine kinase activation by p21-activated kinases (PAKs). J. Biol. Chem. 291, 20042–20054 (2016).
Fauchereau, F. et al. The RhoGAP activity of OPHN1, a new F-actin-binding protein, is negatively controlled by its amino-terminal domain. Mol. Cell. Neurosci. 23, 574–586 (2003).
Schlam, D. et al. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat. Commun. 6, 8623 (2015).
Michaelson, D. et al. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111–126 (2001).
Yonemura, S., Hirao-Minakuchi, K. & Nishimura, Y. Rho localization in cells and tissues. Exp. Cell Res. 295, 300–314 (2004).
Lu, M. S. & Drubin, D. G. Cdc42 GTPase regulates ESCRTs in nuclear envelope sealing and ER remodeling. J. Cell Biol. 219, e201910119 (2020).
Boulter, E. et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat. Cell Biol. 12, 477–483 (2010).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Kraynov, V. S. et al. Localized Rac activation dynamics visualized in living cells. Science 290, 333–337 (2000).
Fritz, R. D. et al. A versatile toolkit to produce sensitive FRET biosensors to visualize signaling in time and space. Sci. Signal. 6, rs12 (2013).
Acknowledgements
The authors thank colleagues who generously provided unpublished images: J. Michaux and E. Munro, University of Chicago; C. Tong and M. Wu, Yale University; A. Michaud, Promega Corp.; M. Graessl, P. Nalbant and L. Dehmelt, University of Duisburg and Technical University of Dortmund; and L. Hoachlander-Hobby, University of Wisconsin–Madison. W.M.B. acknowledges funding from the National Institutes of Health (NIH RO1 GM052932) and the National Science Foundation (NSF 2132606), A.L.M. acknowledges funding from the National Institutes of Health (NIH R01 GM112794) and the National Science Foundation (NSF 1615338), and A.B.G acknowledges funding from the Biotechnology and Biological Sciences Research Council (BB/W013614/1) and the Leverhulme Trust (RPG-2020-220). To the memory of Robert K. Bement, 1932–2023: ‘Er ist geflohen, als wir ihn nicht beobachteten’.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bement, W.M., Goryachev, A.B., Miller, A.L. et al. Patterning of the cell cortex by Rho GTPases. Nat Rev Mol Cell Biol 25, 290–308 (2024). https://doi.org/10.1038/s41580-023-00682-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-023-00682-z