Abstract
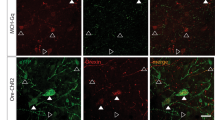
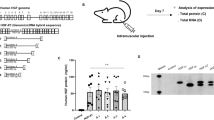
The hormone leptin has been shown to be an afferent signal in a negative-feedback loop regulating body weight, and consequently, the administration of the gene product for the treatment of obesity has recently attracted considerable attention. Leptin is produced by adipocytes in response to increased trigyceride storage, and appears to affect body weight primarily through target cells in the hypothalamus. Although plasma levels of leptin correlate positively with adipose tissue mass in normal humans and animals1,2,3,4, recent studies have shown that obese humans and animals appear to be relatively resistant to the increased plasma levels of leptin1,5. Analysis of the levels of leptin in the cerebrospinal fluid suggests that the uptake of leptin across the blood–brain barrier may be saturable1. Taken together, these results suggest that therapeutic approaches to deliver leptin through the circulation may prove to be problematic. Although recent clinical trials have suggested that peripherally administered leptin might lead to a reduction in body weight in humans6, it is likely that the more effective delivery of leptin to cellular targets within the central nervous system will be necessary in order to fully reveal the therapeutic potential of the gene product. In an effort to provide a means for the delivery of leptin that obviates the need for the gene product to traverse the blood–brain barrier, we have evaluated the use of recombinant adeno-associated vectors to deliver leptin intraventricularly or directly to the hypothalamus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Caro, J.F. et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance [see comments]. Lancet 348, 159–161 (1996).
Considine, R.V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans [see comments]. N. Engl. J. Med. 334, 292–295 (1996).
Frederich, R.C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Maffei, M. et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 (1995).
Halaas, J.L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 94, 8878–8883 (1997).
Heymsfield, S.B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. J. Am Med. Assoc. 282, 1568–1575 (1999).
Simerly, R.B. Anatomical substrates of hypothalamic integration. In The rat nervous system. (ed. Paxinos, G.) 353–372 (Academic Press, San Diego, CA; 1995).
Fisher, K.J. et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 3, 306–312 (1997).
Mandel, R.J. et al. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson's disease. J. Neurosci. 18, 4271–4284 (1998).
Bartlett, J.S., Samulski, R.J. & McCown, T.J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum. Gene Ther. 9, 1181–1186 (1998).
Johansson, C.B. et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25–34 (1999).
Doetsch, F., Caille, I., Lim, D.A., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Morsy, M.A. et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 95, 7866–7871 (1998).
Murphy, J.E. et al. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc. Natl. Acad. Sci. USA 94, 13921–13926 (1997).
McCown, T.J., Xiao, X., Li, J., Breese, G.R. & Samulski, R.J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 713, 99–107 (1996).
Kaplitt, M.G. et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat. Genet. 8, 148–154 (1994).
Mandel, R.J., Spratt, S.K., Snyder, R.O. & Leff, S.E. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson's disease in rats. Proc. Natl. Acad. Sci. USA 94, 14083–14088 (1997).
Snyder, R.O. et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum. Gene Ther. 8, 1891–1900 (1997).
Gundersen, H.J. et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis 96, 857–881 (1988).
Acknowledgements
This work was supported by the Howard Hughes Medical Institute. C.L is a Wenner-Gren Foundation post-doctoral fellow. R.C.M. is currently an equity-holding consultant with AMGEN, Inc. and receives sponsored research support from the company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundberg, C., Jungles, S. & Mulligan, R. Direct delivery of leptin to the hypothalamus using recombinant adeno-associated virus vectors results in increased therapeutic efficacy. Nat Biotechnol 19, 169–172 (2001). https://doi.org/10.1038/84448
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/84448
This article is cited by
-
Overexpression of CART in the PVN Increases Food Intake and Weight Gain in Rats
Obesity (2008)
-
Treatment of human disease by adeno-associated viral gene transfer
Human Genetics (2006)
-
Hypothalamic delivery of doxycycline-inducible leptin gene allows for reversible transgene expression and physiological responses
Gene Therapy (2002)