Abstract
Appropriate responses to an imminent threat brace us for adversities. The ability to sense and predict threatening or stressful events is essential for such adaptive behaviour. In the mammalian brain, one putative stress sensor is the paraventricular nucleus of the thalamus (PVT), an area that is readily activated by both physical and psychological stressors1,2,3. However, the role of the PVT in the establishment of adaptive behavioural responses remains unclear. Here we show in mice that the PVT regulates fear processing in the lateral division of the central amygdala (CeL), a structure that orchestrates fear learning and expression4,5. Selective inactivation of CeL-projecting PVT neurons prevented fear conditioning, an effect that can be accounted for by an impairment in fear-conditioning-induced synaptic potentiation onto somatostatin-expressing (SOM+) CeL neurons, which has previously been shown to store fear memory6. Consistently, we found that PVT neurons preferentially innervate SOM+ neurons in the CeL, and stimulation of PVT afferents facilitated SOM+ neuron activity and promoted intra-CeL inhibition, two processes that are critical for fear learning and expression5,6. Notably, PVT modulation of SOM+ CeL neurons was mediated by activation of the brain-derived neurotrophic factor (BDNF) receptor tropomysin-related kinase B (TrkB). As a result, selective deletion of either Bdnf in the PVT or Trkb in SOM+ CeL neurons impaired fear conditioning, while infusion of BDNF into the CeL enhanced fear learning and elicited unconditioned fear responses. Our results demonstrate that the PVT–CeL pathway constitutes a novel circuit essential for both the establishment of fear memory and the expression of fear responses, and uncover mechanisms linking stress detection in PVT with the emergence of adaptive behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spencer, S. J., Fox, J. C. & Day, T. A. Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res. 997, 234–237 (2004)
Chastrette, N., Pfaff, D. W. & Gibbs, R. B. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 563, 339–344 (1991)
Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H. & Watson, S. J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505 (1995)
Wilensky, A. E., Schafe, G. E., Kristensen, M. P. & LeDoux, J. E. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J. Neurosci. 26, 12387–12396 (2006)
Ciocchi, S. et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010)
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nature Neurosci. 16, 332–339 (2013)
Krout, K. E. & Loewy, A. D. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 428, 475–494 (2000)
Bhatnagar, S. et al. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J. Neurosci. 20, 5564–5573 (2000)
Li, S. & Kirouac, G. J. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 506, 263–287 (2008)
Vertes, R. P. & Hoover, W. B. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–237 (2008)
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007)
Darvas, M. & Palmiter, R. D. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proc. Natl Acad. Sci. USA 106, 14664–14669 (2009)
Do-Monte, F. H., Quinones-Laracuente, K. & Quirk, G. J. A temporal shift in the circuits mediating retrieval of fear memory. Nature http://dx.doi.org/10.1038/nature14030 (this issue)
He, M. et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73, 35–48 (2012)
Haubensak, W. et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010)
Miyamichi, K. et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature 472, 191–196 (2011)
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nature Methods 9, 159–172 (2011)
Park, H. & Poo, M.-M. Neurotrophin regulation of neural circuit development and function. Nature Rev. Neurosci. 14, 7–23 (2013)
Conner, J. M., Lauterborn, J. C., Yan, Q., Gall, C. M. & Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J. Neurosci. 17, 2295–2313 (1997)
He, X.-P. et al. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron 43, 31–42 (2004)
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nature Methods 11, 763–772 (2014)
Rios, M. et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 (2001)
Johnson, P. L., Molosh, A., Fitz, S. D., Truitt, W. A. & Shekhar, A. Orexin, stress, and anxiety/panic states. Prog. Brain Res. 198, 133–161 (2012)
Li, Y. et al. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol. Biochem. Behav. 95, 121–128 (2010)
Zhang, L. et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol. Psychiatry 19, 8–10 (2014)
Mahan, A. L. & Ressler, K. J. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 35, 24–35 (2012)
Li, L. et al. Visualizing the distribution of synapses from individual neurons in the mouse brain. PLoS ONE 5, e11503 (2010)
Acknowledgements
We thank E. Nestler for providing us with the Trkblox/lox mice generated by L.F.P., K. Deisseroth for the AAV-Ef1a-fDIO backbone, E. Valjent for supporting D.D.B.’s work, and members of the Li laboratory for discussions. This work was supported by grants from the National Institutes of Health (NIH) (B.L., L.V.A. and Z.J.H.), the Dana Foundation (B.L.), NARSAD (B.L. and Z.J.H.), Louis Feil Trust (B.L.), the Stanley Family Foundation (B.L. and Z.J.H.), and a Harvey L. Karp Discovery Award (M.A.P.).
Author information
Authors and Affiliations
Contributions
M.A.P. and B.L. designed the study. M.A.P. and V.R. conducted experiments. M.A.P. analysed data. J.T. assisted with the rabies viral tracing experiments. D.D.B. assisted with the BDNF infusion experiments. M.W. made the AAV-fDIO-Cre-GFP virus. M.D. made the CAV2-Cre virus. L.F.P. generated the Trkblox/lox mouse line. M.H. generated the Som-Flp mouse line. L.V.A., R.D.P. and Z.J.H. provided critical reagents and suggestions. M.A.P. and B.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 PVT is activated following both fear conditioning and fear memory retrieval.
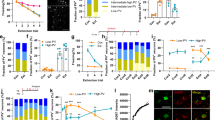
a, A schematic of the experimental design. All mice were killed for the detection of c-Fos at 90 min after the last behavioural session. b, Representative images of c-Fos immunohistochemistry in the pPVT for the five groups indicated in a. c, Quantification of c-Fos expression as c-Fos+ cells per mm2 (post-habituation 1 (mice that were subjected to one session of habituation), 131.17 ± 34.25, n = 4 mice; post-habituation 2 (mice that were subjected to two sessions of habituation), 180.68 ± 30.42, n = 5 mice; post-US (unconditioned stimulus; mice that were only exposed to five foot shocks), 443.3 ± 25.7, n = 3 mice; post-conditioning, 692.61 ± 46.68, n = 4 mice; post-retrieval, 565.51 ± 28.71, n = 3 mice; F(4,14) = 49.3, P < 0.001; P < 0.01, P < 0.001; one-way analysis of variance (ANOVA) followed by Tukey’s test). Data are presented as mean ± s.e.m.
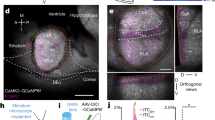
Extended Data Figure 2 pPVT neurons projecting to the BLA and CeL are non-overlapping.
a, A schematic of the approach to simultaneously label BLA- and CeL-projecting pPVT neurons. b, A representative image of the injection sites, where CTB-488 and CTB-555 were injected into the CeL and BLA, respectively. c, Representative images of pPVT cells labelled by CTB-488 (left) and CTB-555 (middle). These two populations were largely non-overlapping (right). Data was replicated in 4 mice.
Extended Data Figure 3 Performance during conditioning.
a–d, Freezing levels during conditioning are shown for mice used in Fig. 1c (a), Fig. 2 (b), Fig. 4c (c), and Fig. 4e (d). a, There was no significant difference in performance among groups (F(2,155) = 0.51, P > 0.05, two-way ANOVA). b, There was no significant difference in performance between saline-treated mice and CNO-treated mice (F(1,70) = 0.43, P > 0.05, two-way ANOVA). c, There was no significant difference in performance between the two groups (F(1,85) = 0.73, P > 0.05, two-way ANOVA). d, There was no significant difference in performance between the two groups (F(1,75) = 0.45, P > 0.05, two-way ANOVA). Data are presented as mean ± s.e.m.
Extended Data Figure 4 The pPVT is required for fear-conditioning-induced synaptic plasticity in the lateral-amygdala–CeLSOM pathway.
a, Top, a schematic of the whole-cell paired recording configuration. A pair of SOM+ and SOM− CeL neurons were simultaneously recorded, and EPSCs were evoked by stimulation of the lateral amygdala. We used the Som-cre;H2b-GFP mice, in which the SOM+ neurons were tagged with H2b–GFP and the pPVT neurons were infected with hM4Di as in Fig. 2a, b. Bottom, a representative image of a slice used for recording, in which a SOM+ (arrow) and an adjacent SOM– (arrowhead) neuron were recorded. b, Sample EPSC traces obtained from the simultaneous paired recording experiment. Naive control mice (left) and fear-conditioned mice treated with either saline (middle) or CNO (right) were used. Saline or CNO was administered 40 min before, and recordings were performed 24 h after conditioning. Top and bottom traces represent EPSCs recorded at +40 mV and −70 mV holding potentials, respectively. c, Quantification of AMPA (left) and NMDA (right) currents (Control, n = 9 pairs (2 mice); ‘Fear, saline’, n = 8 pairs (3 mice); ‘Fear, CNO’, n = 14 pairs (3 mice); P < 0.05, P < 0.001, n.s., non-significant; paired t-test). EPSC values are normalized to the average EPSC value of SOM− cells for each group. d, Quantification of the paired-pulse ratio (PPR) (see Methods) of EPSCs measured at −70 mV (comparing control, ‘Fear, saline’, and ‘Fear, CNO’ groups for SOM+ neurons: P < 0.05, P < 0.01, n.s., non-significant; one-way ANOVA followed by Tukey’s test). Control mice for all experiments were injected with the same viral vectors as the experimental groups. Data are presented as mean ± s.e.m.
Extended Data Figure 5 A different mode of communication in the pPVT–CeL pathway compared with the pPVT–BLA pathway.
a–c, Optogenetic stimulation of pPVT afferents in the BLA drives fast synaptic transmission onto BLA neurons. a, b, Schematics of the experimental approach. c, Sample trace (average of 20) of the synaptic responses onto a BLA neuron following brief (1-s train of 5-Hz, 1-ms pulses) photostimulation of pPVT afferents expressing ChR2. Similar responses were observed in 5 out of 6 BLA neurons recorded. Data was obtained from the same mice as those in Fig. 3d–g. d–f, Slow recovery of the pPVT-driven current in a SOM+ CeL neuron. d, e, Schematics of the experimental approach. f, Sample trace of the synaptic response onto a SOM+ neuron following prolonged (20-s train of 30-Hz, 1-ms pulses) photostimulation of pPVT afferents expressing ChR2, showing slow recovery after stimulus cessation. g–i, Optogenetic stimulation of the pPVT–CeL pathway promotes intra-CeL inhibition. g, Representative traces of IPSCs onto SOM− (black) and SOM+ (red) CeL neurons. Blue bar indicates the 30 Hz photostimulation of pPVT afferents. h, Quantification of IPSC frequency, comparing pre- and post-photostimulation (SOM−, n = 14 neurons (6 mice), P < 0.001, t-test; SOM+, n = 11 neurons (6 mice), P > 0.05, t-test). i, Quantification of IPSC amplitude, comparing pre- and post-photostimulation (n.s., non-significant (P > 0.05), paired t-test). Data are presented as mean ± s.e.m.
Extended Data Figure 6 CeL-projecting neurons in the pPVT express BDNF.
a, A schematic of the experimental approach to retrogradely label CeL-projecting pPVT cells. b, Representative images of pPVT cells, which were labelled by CTB-488 (left) and an antibody recognizing BDNF (middle). CTB-labelled neurons largely overlapped with BDNF-positive somatas (see overlay in right). c, d, BLA-projecting neurons and BDNF-expressing neurons in pPVT are largely non-overlapping. c, A schematic of the method used to label BLA-projecting neurons in the pPVT. d, Representative images of pPVT cells labelled by either CTB-555 (left) or the antibody recognizing BDNF (middle). These two populations were largely non-overlapping (see overlay in right).
Extended Data Figure 7 BDNF/TrkB mediates pPVT–CeL communication.
a, The TrkB receptor is selectively expressed by SOM+ CeL neurons. Top, representative images of the CeL in Som-cre;H2b-GFP mice, showing SOM+ neurons tagged with H2b–GFP (left) and TrkB expression recognized by an antibody (middle). Bottom, higher-magnification images of the boxed area in the top panel. TrkB-labelled cells largely overlap with SOM+ neurons (see overlay on right). b–f, TrkB mediates the pPVT–CeL transmission. b, A schematic of the experimental approach using the Trkblox/lox;Som-Flp mice to: (1) tag SOM+ CeL neurons with mCherry; (2) sparsely infect CeL neurons with GFP–Cre to delete Trkb; and (3) express ChR2 in the pPVT. c, Representative images resulting from the approach in b, showing CeL neurons expressing (from left to right) Cre–GFP, mCherry and TrkB. Neurons that expressed both mCherry and GFP–Cre represent SOM+ neurons in which Trkb was deleted (arrow; see overlay on right), whereas neurons that expressed mCherry, but not GFP–Cre, represent SOM+ neurons with intact Trkb (arrowhead; see overlay on right). d, A schematic of the whole-cell recording configuration. e, Sample traces of synaptic currents in mCherry-only (SOM+,Cre−; red) and mCherry/GFP–Cre double positive (SOM+,Cre+; yellow) neurons in response to prolonged high-frequency stimulation of pPVT afferents. f, Quantification of the synaptic responses (SOM+,Cre−, 8.06 ± 2.58 pA, n = 7 neurons (3 mice); SOM+,Cre+, 2.10 ± 0.76 pA, n = 7 neurons (3 mice); P < 0.05, t-test). g–j, The pPVT input to the CeL promotes intra-CeL inhibition through BDNF/TrkB signalling. g, Representative traces of IPSCs recorded from SOM− CeL neurons in response to the 30 Hz photostimulation (blue bars) of pPVT afferents, in the control condition (top panel) or in the presence of the BDNF scavenger TrkB–Fc (bottom panel). h, Quantification of the frequency of IPSCs recorded from SOM− CeL neurons (comparing pre- and post-photostimulation: control, n = 14 neurons (6 mice; repetition of data from the SOM– cells in Extended Data Fig. 5h), P < 0.001, paired t-test; TrkB–Fc, n = 17 neurons (2 mice), P > 0.05, paired t-test). i, Representative traces showing the effect of BDNF bath application on spontaneous IPSCs recorded from CeL neurons. j, Quantification of the effect of BDNF on sIPSC frequency. Black line indicates the timing of BDNF application (n = 7; P < 0.05 comparing baseline and BDNF application, paired t-test). Data are presented as mean ± s.e.m.
Extended Data Figure 8 Characterization of the AAV-fDIO-CreGFP.
a, A schematic of the experimental approach to selectively target SOM+ CeL neurons in the Som-Flp mice with the AAV-fDIO-Cre-GFP. b, Representative images of CeL neurons expressing Cre–GFP (left), and PKC-δ+ CeL neurons (as a surrogate for SOM− neurons) that were recognized by an antibody (middle). In the bottom panels are high magnification images of the boxed region in the top panel. These two cell populations were largely non-overlapping (see overlay on right), indicating that the AAV-fDIO-Cre-GFP selectively infects SOM+ neurons (data from one mouse). c, A schematic of the experimental approach to test the function of AAV-fDIO-Cre-GFP, whereby the CeL of Som-Flp mice was injected with a mixture of AAV-fDIO-Cre-GFP and AAV-DIO-hM4Di-mCherry. As the latter virus expresses mCherry in a Cre-dependent manner, observation of selective mCherry expression in GFP+ neurons would indicate that the AAV-fDIO-Cre-GFP is effective. d, Sample images of CeL neurons expressing Cre–GFP (left) and mCherry (middle). In the bottom panels are high magnification images of the boxed region in the top panel. Essentially, all mCherry+ neurons co-expressed GFP (see overlay on right), indicating selective expression of Cre by the GFP-labelled cells (data from one mouse).
Extended Data Figure 9 BDNF/TrkB regulates synaptic plasticity onto SOM+ CeL neurons.
a–d, Selective deletion of Trkb in SOM+ CeL neurons impairs fear-conditioning-induced synaptic plasticity. a, A schematic of the experimental approach to specifically delete Trkb in SOM+ CeL neurons. b, Representative traces of mEPSCs recorded from SOM+ CeL neurons in which Trkb was deleted, in naive control (top) and fear-conditioned (bottom) mice. c, d, Deletion of Trkb blocked the fear conditioning-induced increase in mEPSC frequency (c) (control, n = 19 neurons (3 mice); fear, n = 18 neurons (3 mice); P > 0.05, t-test), but not amplitude (d) (control, n = 19 neurons (3 mice); fear, n = 18 neurons (3 mice); P < 0.05, t-test). e–g, BDNF induces long-term potentiation at lateral-amygdala–CeLSOM synapses. e, A schematic of the whole-cell recording configuration. f, Top, sample EPSC traces recorded before (pre-BDNF) and after (post-BDNF) bath application of BDNF. Bottom, summary plot showing the effect of BDNF on EPSC peak amplitude, for which the first peak in the paired pulse was measured and normalized to the baseline (that is, the average pre-BDNF amplitude). BDNF significantly enhanced EPSC amplitude (pre-BDNF, 98 ± 1.74%, post-BDNF, 146 ± 17.6%, n = 6 neurons (3 mice), P < 0.05, paired t-test). g, BDNF application decreased the paired-pulse ratio (see Methods) of the EPSCs (pre-BDNF, 1.17 ± 0.18; post-BDNF, 0.80 ± 0.09; n = 6 neurons (3 mice), P < 0.05, paired t-test). Data are presented as mean ± s.e.m.
Extended Data Figure 10 Exogenous application of BDNF in the CeL increases the excitability of SOM+ neurons and elicits an unconditioned freezing response.
a–d, BDNF increases the excitability of SOM+ CeL neurons. a, A schematic of the experimental approach, in which photostimulation was used to assess the excitability of SOM+ CeL neurons expressing ChR2. b, Sample traces of photostimulation-evoked spikes recorded in cell-attached mode, before (baseline; left) and after (right) bath application of BDNF (100 ng ml−1). Light intensity was adjusted to evoke spikes with ∼50% probability at baseline. c, A sample recording, in which the spike probability of a SOM+ CeL neuron was followed before, during and after BDNF application. d, Quantification of the effect of BDNF on spike probability (baseline, 0.50 ± 0.02; BDNF, 0.74 ± 0.08; n = 8 neurons (4 mice), P < 0.05, paired t-test). e, f, Infusion of BDNF into the CeL elicits an unconditioned freezing response. e, Drawing of the cannula sites. Each dot denotes where the tip of the injection cannula was located in each mouse. f, Quantification of freezing levels following CeL infusion of saline and BDNF (saline, 2.93 ± 1.84%; BDNF, 32.22 ± 9.19%; n = 6 mice, P < 0.05, paired t-test). Data are presented as mean ± s.e.m.
Supplementary information
Saline infusion into CeL
This video (sped up 4 times) shows a naïve mouse exploring the conditioning box immediately after bilateral infusion of saline vehicle into CeL on Day 1. (MP4 24620 kb)
BDNF infusion into CeL
This video (sped up 4 times) shows the same mouse as that in Supplementary Video 1 immediately after bilateral infusion of BDNF into CeL on Day 2. Robust freezing-like behavior can be observed. (MP4 24689 kb)
Rights and permissions
About this article
Cite this article
Penzo, M., Robert, V., Tucciarone, J. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015). https://doi.org/10.1038/nature13978
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13978
This article is cited by
-
Social buffering in rats reduces fear by oxytocin triggering sustained changes in central amygdala neuronal activity
Nature Communications (2024)
-
Direct paraventricular thalamus-basolateral amygdala circuit modulates neuropathic pain and emotional anxiety
Neuropsychopharmacology (2024)
-
Behavioral phenotype, intestinal microbiome, and brain neuronal activity of male serotonin transporter knockout mice
Molecular Brain (2023)
-
Plasticity of neuronal dynamics in the lateral habenula for cue-punishment associative learning
Molecular Psychiatry (2023)
-
Anxiety in synucleinopathies: neuronal circuitry, underlying pathomechanisms and current therapeutic strategies
npj Parkinson's Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.