Abstract
Permafrost contains about 50% of the global soil carbon1. It is thought that the thawing of permafrost can lead to a loss of soil carbon in the form of methane and carbon dioxide emissions2,3. The magnitude of the resulting positive climate feedback of such greenhouse gas emissions is still unknown3 and may to a large extent depend on the poorly understood role of microbial community composition in regulating the metabolic processes that drive such ecosystem-scale greenhouse gas fluxes. Here we show that changes in vegetation and increasing methane emissions with permafrost thaw are associated with a switch from hydrogenotrophic to partly acetoclastic methanogenesis, resulting in a large shift in the δ13C signature (10–15‰) of emitted methane. We used a natural landscape gradient of permafrost thaw in northern Sweden4,5 as a model to investigate the role of microbial communities in regulating methane cycling, and to test whether a knowledge of community dynamics could improve predictions of carbon emissions under loss of permafrost. Abundance of the methanogen Candidatus ‘Methanoflorens stordalenmirensis’6 is a key predictor of the shifts in methane isotopes, which in turn predicts the proportions of carbon emitted as methane and as carbon dioxide, an important factor for simulating the climate feedback associated with permafrost thaw in global models3,7. By showing that the abundance of key microbial lineages can be used to predict atmospherically relevant patterns in methane isotopes and the proportion of carbon metabolized to methane during permafrost thaw, we establish a basis for scaling changing microbial communities to ecosystem isotope dynamics. Our findings indicate that microbial ecology may be important in ecosystem-scale responses to global change.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tarnocai, C. et al. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 23, GB2023 (2009)
Schuur, E. A. G. et al. Expert assessment of vulnerability of permafrost carbon to climate change. Clim. Change 119, 359–374 (2013)
Ciais, P. et al. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, 2013)
Johansson, T. et al. Decadal vegetation changes in a northern peatland, greenhouse gas fluxes and net radiative forcing. Glob. Change Biol. 12, 2352–2369 (2006)
Christensen, T. R. et al. Thawing sub-arctic permafrost: effects on vegetation and methane emissions. Geophys. Res. Lett. 31, L04501 (2004)
Mondav, R. et al. Discovery of a novel methanogen in thawing permafrost. Nature Commun. 5, 3212, http://dx.doi.org/10.1038/ncomms4212 (14 February 2014)
Melton, J. R. et al. Present state of global wetland extent and wetland methane modelling: conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 10, 753–788 (2013)
Jorgenson, M. T., Racine, C. H., Walters, J. C. & Osterkamp, T. E. Permafrost degradation and ecological changes associated with a warming climate in central Alaska. Clim. Change 48, 551–579 (2001)
Hodgkins, S. B. et al. Changes in peat chemistry associated with permafrost thaw increase greenhouse gas production. Proc. Natl Acad. Sci. USA 111, 5819–5824 (2014)
Olefeldt, D., Turetsky, M. R., Crill, P. M. & McGuire, A. D. Environmental and physical controls on northern terrestrial methane emissions across permafrost zones. Glob. Change Biol. 19, 589–603 (2012)
Lee, H., Schuur, E. A. G., Inglett, K. S., Lavoie, M. & Chanton, J. P. The rate of permafrost carbon release under aerobic and anaerobic conditions and its potential effects on climate. Glob. Change Biol. 18, 515–527 (2012)
Mackelprang, R. et al. Metagenomic analysis of a permafrost microbial community reveals a rapid response to thaw. Nature 480, 368–371 (2011)
Riley, W. J. et al. Barriers to predicting changes in global terrestrial methane fluxes: analyses using CLM4Me, a methane biogeochemistry model integrated in CESM. Biogeosciences 8, 1925–1953 (2011)
Koven, C. D. et al. Permafrost carbon-climate feedbacks accelerate global warming. Proc. Natl Acad. Sci. USA 108, 14769–14774 (2011)
Turetsky, M. R., Wieder, R. K. & Vitt, D. H. Boreal peatland C fluxes under varying permafrost regimes. Soil Biol. Biochem. 34, 907–912 (2002)
Bäckstrand, K. et al. Annual carbon gas budget for a subarctic peatland, northern Sweden. Biogeosciences 7, 95–108 (2010)
Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K. & Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 19, 1325–1346 (2013)
Hornibrook, E. R. C. & Bowes, H. L. Trophic status impacts both the magnitude and stable carbon isotope composition of methane flux from peatlands. Geophys. Res. Lett. 34, 2–6 (2007)
Chanton, J. P., Chaser, L. C., Glaser, P. & Siegel, D. in Stable Isotopes and Biosphere–Atmosphere Interactions (eds Flanagan, L. B., Ehleringer, J. R. & Pataki, D. E. ) 85–105 (Elsevier, 2005)
Whiticar, M. J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 161, 291–314 (1999)
Popp, T. J., Chanton, J. P., Whiting, G. J. & Grant, N. Methane stable isotope distribution at a Carex dominated fen in North Central Alberta. Glob. Biogeochem. Cycles 13, 1063–1077 (1999)
Whiticar, M. J., Faber, E. & Schoel, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—isotope evidence. Geochim. Cosmochim. Acta 50, 693–709 (1986)
Ferry, J. G. How to make a living by exhaling methane. Annu. Rev. Microbiol. 64, 453–473 (2010)
Liu, Y. & Whitman, W. B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. NY Acad. Sci. 1125, 171–189 (2008)
Hornibrook, E. R. C., Longstaffe, F. J. & Fyfe, W. S. Spatial distribution of microbial methane production pathways in temperate zone wetland soils: stable carbon and hydrogen isotope evidence. Geochim. Cosmochim. Acta 61, 745–753 (1997)
Wania, R. et al. Present state of global wetland extent and wetland methane modelling: methodology of a model inter-comparison project (WETCHIMP). Geoscient. Model Devel. 6, 617–641 (2013)
Wieder, W. R., Bonan, G. B. & Allison, S. D. Global soil carbon projections are improved by modelling microbial processes. Nature Clim. Change 3, 909–912 (2013)
Kai, F. M., Tyler, S. C., Randerson, J. T. & Blake, D. R. Reduced methane growth rate explained by decreased Northern Hemisphere microbial sources. Nature 476, 194–197 (2011)
Bousquet, P. et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature 443, 439–443 (2006)
Hines, M. E., Duddleston, K. N., Rooney-Varga, J. N., Fields, D. & Chanton, J. P. Uncoupling of acetate degradation from methane formation in Alaskan wetlands: connections to vegetation distribution. Glob. Biogeochem. Cycles 22, 1–12 (2008)
Payette, S. Accelerated thawing of subarctic peatland permafrost over the last 50 years. Geophys. Res. Lett. 31, 1–4 (2004)
O’Donnell, J. a. et al. The effects of permafrost thaw on soil hydrologic, thermal, and carbon dynamics in an Alaskan peatland. Ecosystems 15, 213–229 (2012)
Vitt, D. H., Halsey, L. A. & Zoltai, S. C. The changing landscape of Canada’s western boreal forest: the current dynamics of permafrost. Can. J. For. Res. 30, 283–287 (2000)
Quinton, W. L., Hayashi, M. & Chasmer, L. E. Permafrost-thaw-induced land-cover change in the Canadian subarctic: implications for water resources. Hydrol. Processes 25, 152–158 (2011)
Zoltai, S. C. Cyclic development of permafrost in the peatlands of Northwestern Alberta, Canada. Arct. Alp. Res. 25, 240–246 (1993)
Camill, P. & Clark, J. S. Climate change disequilibrium of boreal permafrost peatlands caused by local processes. Am. Nat. 151, 207–222 (1998)
Dyke, L. D. & Sladen, W. E. Permafrost and peatland evolution in the Northern Hudson Bay Lowland, Manitoba. Arctic 63, 429–441 (2010)
Whiticar, M. J. & Faber, E. Methane oxidation in sediment and water column environments—isotopic evidence. Org. Geochem. 10, 759–768 (1986)
Conrad, R. Quantification of methanogenic pathways using stable carbon isotopic signatures: a review and a proposal. Org. Geochem. 36, 739–752 (2005)
Bäckstrand, K., Crill, P. M., Mastepanov, M., Christensen, T. R. & Bastviken, D. Non-methane volatile organic compound flux from a subarctic mire in Northern Sweden. Tellus B Chem. Phys. Meterol. 60, 226–237 (2008)
Bubier, J. L., Crill, P. M., Mosedale, A., Frolking, S. & Linder, E. Peatland responses to varying interannual moisture conditions as measured by automatic CO2 chambers. Glob. Biogeochem. Cycles 17, 1066, http://dx.doi.org/10.1029/2002GB001946 (2003)
Santoni, G. W. et al. Mass fluxes and isofluxes of methane (CH4) at a New Hampshire fen measured by a continuous wave quantum cascade laser spectrometer. J. Geophys. Res. 117, D10301 (2012)
Werle, P., Mücke, R. & Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 139, 131–139 (1993)
Bäckstrand, K., Crill, P. M., Mastepanov, M., Christensen, T. R. & Bastviken, D. Total hydrocarbon flux dynamics at a subarctic mire in northern Sweden. J. Geophys. Res. 113, G03026, http://dx.doi.org/10.1029/2008JG000703 (2008)
Pataki, D. E. The application and interpretation of Keeling plots in terrestrial carbon cycle research. Glob. Biogeochem. Cycles 17, 1022, http://dx.doi.org/10.1029/2001GB001850 (2003)
Keeling, C. D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta 13, 322–334 (1958)
Keeling, C. D. The concentration and isotopic abundances of carbon dioxide in rural and marine air. Geochim. Cosmochim. Acta 24, 277–298 (1960)
R Development Core Team. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012)
Corbett, J. E. et al. Partitioning pathways of CO2 production in peatlands with stable carbon isotopes. Biogeochemistry 114, 327–340 (2013)
Chanton, J. P., Fields, D. & Hines, M. E. Controls on the hydrogen isotopic composition of biogenic methane from high-latitude terrestrial wetlands. J. Geophys. Res. 111, 1–9 (2006)
Bragg, L., Stone, G., Imelfort, M., Hugenholtz, P. & Tyson, G. W. Fast, accurate error-correction of amplicon pyrosequences. Nature Methods 9, 425–426 (2012)
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of proteins or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006)
Altschul, H. J. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997)
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7, 335–336 (2010)
Ludwig, W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004)
Conrad, R. The global methane cycle: recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 1, 285–292 (2009)
Tans, P. P. A note on isotopic ratios and the global atmospheric methane budget. Glob. Biogeochem. Cycles 11, 77–81 (1997)
Acknowledgements
We thank the Abisko Scientific Research Station for infrastructure and logistical support; T. Logan and N. Rakos for their assistance in the field; and S. Wofsy and S. Frolking for feedback on a draft of this paper. This work was supported by the US Department of Energy Office of Biological and Environmental Research (award DE-SC0004632), and by the University of Arizona Technology and Research Initiative Fund, through the Water, Environmental and Energy Solutions Initiative. R.M. was supported by an Australian Postgraduate Award Scholarship.
Author information
Authors and Affiliations
Contributions
S.R.S., V.I.R., P.M.C., J.C. and G.W.T. designed the study. C.K.M., S.B.H., R.A.W., P.M.C., J.C. and S.R.S. designed and/or performed flux/porewater/isotope measurements and laboratory incubations. C.K.M., B.J.W., R.M., E.-H.K., S.R.S., V.I.R. and G.W.T. designed and/or performed analyses integrating bioinformatics and biogeochemistry. C.K.M., V.I.R. and S.R.S. wrote the paper in consultation with B.J.W., S.B.H., J.C., P.M.C., E.-H.K., R.M. and G.W.T.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Expected and observed relationships between the δD and δ13C content of porewater CH4.
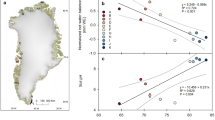
The thick grey arrow shows the expected pattern in H and C isotopes of CH4 when variations are caused by shifts between acetoclastic (lower right) and hydrogenotrophic (upper left) production. The thin black arrows pointing to the upper right indicate the expected pattern in H and C isotopes of CH4 when variations are caused by changes in CH4 oxidation19. The points are observed isotopic compositions of samples collected between July and October 2011 at the partly thawed Sphagnum and fully thawed Eriophorum sites; site averages are shown with error bars (error bars represent s.e.m.; n = 13 (Sphagnum) and 20 (Eriophorum)). Although the scatter allows for some variation in both production and oxidation, the average Eriophorum porewater CH4 had significantly more 13C and less D relative to Sphagnum porewater (Hotelling’s T2 test, P = 0.0001, n = 33), indicating that the overall inter-site isotopic differences were due mostly to differences in the CH4 production pathway rather than to differences in CH4 oxidation. Additionally, in August there was a significant negative relationship between δ13C-CH4 and δD-CH4 of porewater samples collected across sites (dashed line, linear regression, R2 = 0.5, P < 0.02, n = 12). Note that on the vertical axis δD-H2O has been subtracted from δD-CH4 to correct for the effect of δD exchange between H2O and CH4 (refs 20, 38, 50).
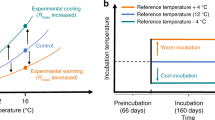
Extended Data Figure 2 Simulations, using high and low temperature and C release scenarios, of the effect of CH4 release from thawing permafrost on atmospheric δ13C-CH4.
a, Scenarios of permafrost C release due to thaw (red bounding lines, high temperature; orange bounding lines, low temperature; the range in each case is defined by high and low C release scenarios). b, Impact on atmospheric methane mixing ratios (assuming that 2.3% of released C is emitted as methane). c, Impact of the high climate change scenario on atmospheric methane isotopes, assuming Eriophorum-like emissions (blue bounding lines, δ13C ≈ −65‰), or assuming Sphagnum-like emissions (green bounding lines, δ13C ≈ −80‰). d, As in c, except for the low climate change scenario. In c and d, dotted horizontal lines indicate the detection limit for CH4 isotopes28.
Supplementary information
Supplementary Data
Operational taxonomic unit (OTU) table from 16S rRNA gene amplicon analysis. Each row represents an OTU. The first set of columns show the number of that 16S rRNA gene amplicon found in each sample. The rightmost columns show the taxonomy of that OTU predicted with BLAST. The samples presented in this study represent a subset of a larger sampling campaign (eg. Mondav et al 2014) therefore not all OTU's identified in the larger sample-set are present in this table. (XLS 9134 kb)
Rights and permissions
About this article
Cite this article
McCalley, C., Woodcroft, B., Hodgkins, S. et al. Methane dynamics regulated by microbial community response to permafrost thaw. Nature 514, 478–481 (2014). https://doi.org/10.1038/nature13798
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13798
This article is cited by
-
Boreal–Arctic wetland methane emissions modulated by warming and vegetation activity
Nature Climate Change (2024)
-
Multifarious Responses of Forest Soil Microbial Community Toward Climate Change
Microbial Ecology (2023)
-
Effects of microbial-converted ancient permafrost organic carbon on the growth and reproduction of Daphnia magna
Oecologia (2023)
-
Microbial iron cycling during palsa hillslope collapse promotes greenhouse gas emissions before complete permafrost thaw
Communications Earth & Environment (2022)
-
Improved global wetland carbon isotopic signatures support post-2006 microbial methane emission increase
Communications Earth & Environment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.