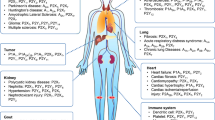
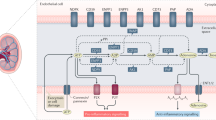
Abstract
Inflammatory conditions are associated with the extracellular release of nucleotides, particularly ATP. In the extracellular compartment, ATP predominantly functions as a signalling molecule through the activation of purinergic P2 receptors. Metabotropic P2Y receptors are G-protein-coupled, whereas ionotropic P2X receptors are ATP-gated ion channels. Here we discuss how signalling events through P2 receptors alter the outcomes of inflammatory or infectious diseases. Recent studies implicate a role for P2X/P2Y signalling in mounting appropriate inflammatory responses critical for host defence against invading pathogens or tumours. Conversely, P2X/P2Y signalling can promote chronic inflammation during ischaemia and reperfusion injury, inflammatory bowel disease or acute and chronic diseases of the lungs. Although nucleotide signalling has been used clinically in patients before, research indicates an expanding field of opportunities for specifically targeting individual P2 receptors for the treatment of inflammatory or infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 May 2014
An incorrect URL was shown for http://www.clinicaltrials.gov/ in the print version of this Review, and has been corrected in the online version.
References
Khakh, B. S. & Burnstock, G. The double life of ATP. Sci. Am. 301, 84–92 (2009)
Junger, W. G. Immune cell regulation by autocrine purinergic signalling. Nature Rev. Immunol. 11, 201–212 (2011)Provides a comprehensive overview of the functional roles of purinergic signalling events on cells of the adaptive and innate immune systems.
Fredholm, B. & Verkhratsky, A. Purines — 80 years and very much alive. Acta Physiol. 199, 91–92 (2010)
Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nature Rev. Drug. Discov. 7, 575–590 (2008)
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012)
Eltzschig, H. K., Macmanus, C. F. & Colgan, S. P. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc. Med. 18, 103–107 (2008)
Eltzschig, H. K. et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 99, 1100–1108 (2006)
Chekeni, F. B. et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010)Identifies a functional role for pannexin-mediated ATP release from cells undergoing apoptosis as a ‘find-me’ signal for phagocytes.
Faigle, M., Seessle, J., Zug, S., El Kasmi, K. C. & Eltzschig, H. K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3, e2801 (2008)
Lazarowski, E. R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8, 359–373 (2012)
Abbracchio, M. P. et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341 (2006)
Jacobson, K. A., Balasubramanian, R., Deflorian, F. & Gao, Z. G. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal. 8, 419–436 (2012)Provides an update on the medicinal chemistry and pharmacology of the different subtypes of adenosine receptors and P2Y receptors, including recent advances in the identification and characterization of selective ligands.
Soulet, C. et al. Gi-dependent and -independent mechanisms downstream of the P2Y12 ADP-receptor. J. Thromb. Haemost. 2, 135–146 (2004)
Hardy, A. R. et al. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 105, 3552–3560 (2005)
von Kugelgen, I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 110, 415–432 (2006)
Harden, T. K., Sesma, J. I., Fricks, I. P. & Lazarowski, E. R. Signalling and pharmacological properties of the P2Y receptor. Acta Physiol. 199, 149–160 (2010)
Hawking, F. Suramin: with special reference to onchocerciasis. Adv. Pharmacol. Chemother. 15, 289–322 (1978)
Voogd, T. E., Vansterkenburg, E. L., Wilting, J. & Janssen, L. H. Recent research on the biological activity of suramin. Pharmacol. Rev. 45, 177–203 (1993)
Ratjen, F. et al. Long term effects of denufosol tetrasodium in patients with cystic fibrosis. J. Cyst. Fibros. 11, 539–549 (2012)
Rieg, T. et al. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J. 21, 3717–3726 (2007)
Horckmans, M. et al. Gene deletion of P2Y4 receptor lowers exercise capacity and reduces myocardial hypertrophy with swimming exercise. Am. J. Physiol. Heart. Circ. Physiol. 303, H835–H843 (2012)
Knowles, M. R., Clarke, L. L. & Boucher, R. C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 325, 533–538 (1991)
Parr, C. E. et al. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl Acad. Sci. USA 91, 3275–3279 (1994)
Davis, S. D. & Ferkol, T. Identifying the origins of cystic fibrosis lung disease. N. Engl. J. Med. 368, 2026–2028 (2013)
Burnstock, G., Brouns, I., Adriaensen, D. & Timmermans, J. P. Purinergic signaling in the airways. Pharmacol. Rev. 64, 834–868 (2012)
Kellerman, D. et al. Denufosol: a review of studies with inhaled P2Y2 agonists that led to Phase 3. Pulm. Pharmacol. Ther. 21, 600–607 (2008)
Deterding, R. R. et al. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am. J. Respir. Crit. Care Med. 176, 362–369 (2007)
Gendaszewska-Darmach, E. & Kucharska, M. Nucleotide receptors as targets in the pharmacological enhancement of dermal wound healing. Purinergic Signal. 7, 193–206 (2011)
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009)
Chen, Y. et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci. Signal 3, ra45 (2010)
Chen, Y. et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 (2006)Shows that human neutrophils release ATP from the leading edge of the cell surface to amplify chemotactic signals and direct cell orientation by feedback through P2Y 2 R as a mechanism of purinergic chemotaxis.
Myrtek, D. & Idzko, M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 3, 5–11 (2007)
Ferrari, D. et al. Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J. Leukoc. Biol. 79, 7–15 (2006)
Kronlage, M. et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal 3, ra55 (2010)
Ben Yebdri, F., Kukulski, F., Tremblay, A. & Sevigny, J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur. J. Immunol. 39, 2885–2894 (2009)
Kobayashi, T., Kouzaki, H. & Kita, H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J. Immunol. 184, 6350–6358 (2010)
Geary, C. et al. Increased susceptibility of purinergic receptor-deficient mice to lung infection with Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 289, L890–L895 (2005)
Inoue, Y., Chen, Y., Hirsh, M. I., Yip, L. & Junger, W. G. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock 30, 173–177 (2008)
Ayata, C. K. et al. Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 143, 1620–1629 (2012)
Lommatzsch, M. et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 928–934 (2010)
Cicko, S. et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J. Immunol. 185, 688–697 (2010)
Idzko, M. et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nature Med. 13, 913–919 (2007)Shows that allergen challenge causes acute accumulation of ATP in the airways of asthmatic subjects or in mice with experimentally induced asthma, and further implicates purinergic signalling as a key mediator in allergen-driven lung inflammation.
Muller, T. et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 65, 1545–1553 (2010)
Weber, F. C. et al. Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J. Exp. Med. 207, 2609–2619 (2010)Identifies P2X 7 R as a crucial receptor for extracellular ATP released in skin in response to contact allergens, and triggering of contact hypersensitivity.
Idzko, M. et al. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br. J. Pharmacol. 138, 1244–1250 (2003)
Kouzaki, H., Iijima, K., Kobayashi, T., O’Grady, S. M. & Kita, H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 186, 4375–4387 (2011)
Kunzli, B. M. et al. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G223–G230 (2007)
Vieira, R. P. et al. Purinergic receptor type 6 contributes to airway inflammation and remodeling in experimental allergic airway inflammation. Am. J. Respir. Crit. Care Med. 184, 215–223 (2011)
Grbic, D. M., Degagne, E., Langlois, C., Dupuis, A. A. & Gendron, F. P. Intestinal inflammation increases the expression of the P2Y6 receptor on epithelial cells and the release of CXC chemokine ligand 8 by UDP. J. Immunol. 180, 2659–2668 (2008)
Riegel, A. K. et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 117, 2548–2555 (2011)
Zhang, Z. et al. P2Y6 agonist uridine 5′-diphosphate promotes host defense against bacterial infection via monocyte chemoattractant protein-1-mediated monocytes/macrophages recruitment. J. Immunol. 186, 5376–5387 (2011)
Idzko, M. et al. Characterization of the biological activities of uridine diphosphate in human dendritic cells: influence on chemotaxis and CXCL8 release. J. Cell. Physiol. 201, 286–293 (2004)
Ferrari, D. et al. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 486, 217–224 (2000)
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007)Demonstrates that P2Y 6 R is upregulated when neurons are damaged, and implicates its signalling function as a sensor for phagocytosis by sensing diffusible UDP signals, a novel pathway mediating microglial phagocytosis.
Grbic, D. M. et al. P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in epithelial cells. Inflamm. Bowel Dis. 18, 1456–1469 (2012)
Guns, P. J., Hendrickx, J., Van Assche, T., Fransen, P. & Bult, H. P2Y receptors and atherosclerosis in apolipoprotein E-deficient mice. Br. J. Pharmacol. 159, 326–336 (2010)
Semple, J. W., Italiano, J. E., Jr. & Freedman, J. Platelets and the immune continuum. Nature Rev. Immunol. 11, 264–274 (2011)
Muhlestein, J. B. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb. Haemost. 103, 71–82 (2010)
Li, D. et al. Roles of purinergic receptor P2Y, G protein-coupled 12 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32, e81–e89 (2012)
Yashiro, K. et al. Involvement of platelet activation by P2Y12 receptor in the development of transplant arteriosclerosis in mice. Transplantation 87, 660–667 (2009)
Paruchuri, S. et al. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J. Exp. Med. 206, 2543–2555 (2009)
Neves, J. S., Radke, A. L. & Weller, P. F. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J. Allergy Clin. Immunol. 125, 477–482 (2010)
Ben Addi, A., Cammarata, D., Conley, P. B., Boeynaems, J. M. & Robaye, B. Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J. Immunol. 185, 5900–5906 (2010)
Bunyavanich, S., Boyce, J. A., Raby, B. A. & Weiss, S. T. Gene-by-environment effect of house dust mite on purinergic receptor P2Y12 (P2RY12) and lung function in children with asthma. Clin. Exp. Allergy 42, 229–237 (2012)
Surprenant, A. & North, R. A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 (2009)
Khakh, B. S. & North, R. A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76, 51–69 (2012)
Khakh, B. S. & North, R. A. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527–532 (2006)Describes P2X signalling beyond its function in the autonomic nervous system, with particular focus on its key roles in afferent signalling, chronic pain and autocrine loops of endothelial and epithelial cells.
Kawate, T., Michel, J. C., Birdsong, W. T. & Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 460, 592–598 (2009)Presents the crystal structure of zebrafish P2X 4 R in its closed, resting state, including definition of the locations of three non-canonical, intersubunit ATP-binding sites, and evidence suggesting that ATP binding promotes subunit rearrangement and ion channel opening.
Alberto, A. V. et al. Is pannexin the pore associated with the P2X7 receptor? Naunyn Schmiedebergs Arch. Pharmacol. 386, 775–787 (2013)
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A. & Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738 (1996)
Schwarz, N. et al. Alternative splicing of the N-terminal cytosolic and transmembrane domains of P2X7 controls gating of the ion channel by ADP-ribosylation. PLoS One 7, e41269 (2012)
North, R. A. & Jarvis, M. F. P2X receptors as drug targets. Mol. Pharmacol. 83, 759–769 (2013)
Mulryan, K. et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403, 86–89 (2000)
Yan, D. et al. Mutation of the ATP-gated P2X2 receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl Acad. Sci. USA 110, 2228–2233 (2013)
Cockayne, D. A. et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015 (2000)
Coutinho-Silva, R., Knight, G. E. & Burnstock, G. Impairment of the splenic immune system in P2X2/P2X3 knockout mice. Immunobiology 209, 661–668 (2005)
Falzoni, S. et al. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J. Clin. Invest. 95, 1207–1216 (1995)
Coutinho-Silva, R. & Ojcius, D. M. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 14, 1271–1277 (2012)
Stagg, J. & Smyth, M. J. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29, 5346–5358 (2010)
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nature Med. 15, 1170–1178 (2009)
Muller, T. et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am. J. Respir. Cell Mol. Biol. 44, 456–464 (2011)
Wilhelm, K. et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nature Med. 16, 1434–1438 (2010)Reveals the relevance of ATP-induced activation of P2X 7 R for graft-versus-host disease development, indicating that the physiological metabolite ATP must be recognized as an endogenous danger signal that has detrimental effects when released into the extracellular space after tissue damage through the activation of recipient antigen-presenting cells.
Killeen, M. E., Ferris, L., Kupetsky, E. A., Falo, L., Jr. & Mathers, A. R. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J. Immunol. 190, 4324–4336 (2013)
Manthei, D. M. et al. Protection from asthma in a high-risk birth cohort by attenuated P2X7 function. J. Allergy Clin. Immunol. 130, 496–502 (2012)
Atarashi, K. et al. ATP drives lamina propria TH17 cell differentiation. Nature 455, 808–812 (2008)
Gulbransen, B. D. et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nature Med. 18, 600–604 (2012)Provides evidence that activation of neuronal P2X 7 R through pannexin 1 underlies neuron death and the subsequent development of abnormal gut motility in IBD, suggesting that this pathway could be targeted to ameliorate the progression of gut dysmotility during intestinal inflammation.
Kurashima, Y. et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nature Commun. 3, 1034 (2012)
Lucattelli, M. et al. P2X7 receptor signalling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 44, 423–429 (2010)
Zimmermann, H., Zebisch, M. & Strater, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502 (2012)Summarizes what is known about the cellular and molecular functions of ATP-hydrolysing ectonucleotidases.
Kohler, D. et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116, 1784–1794 (2007)
Pinsky, D. J. et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 109, 1031–1040 (2002)
Flogel, U. et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 4, 146ra108 (2012)Here the authors developed a selective ADORA2A agonist that requires the presence of CD73 to become activated. This compound suppresses joint inflammation in experimental rheumatoid arthritis, while avoiding ADORA2A-mediated vasodilation.
Ohta, A. & Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 (2001)First study to provide genetic evidence for a non-redundant role for the P1 adenosine receptor ADORA2A in the physiological negative feedback mechanism for limitation and termination of both tissue-specific and systemic inflammatory responses.
Sitkovsky, M. V. et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 22, 657–682 (2004)
Colgan, S. P. & Eltzschig, H. K. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 74, 153–175 (2012)
Friedman, D. J. et al. CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl Acad. Sci. USA 106, 16788–16793 (2009)Provides evidence that CD39 deficiency exacerbates murine colitis and suggests that CD39 polymorphisms are associated with IBD in humans.
Eckle, T. et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J. Immunol. 178, 8127–8137 (2007)
Chevalier, M. F. & Weiss, L. The split personality of regulatory T cells in HIV infection. Blood 121, 29–37 (2013)
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007)Identifies CD39 and CD73 as surface markers of T regs and implicates extracellular adenosine generation in an autocrine signalling loop critical for the suppressor functions of T regs.
Ehrentraut, H. et al. CD73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J. 27, 2207–2219 (2013)
Nikolova, M. et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 7, e1002110 (2011)
Beldi, G. et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology 48, 841–852 (2008)
Sansom, F. M. et al. A bacterial ecto-triphosphate diphosphohydrolase similar to human CD39 is essential for intracellular multiplication of Legionella pneumophila. Cell Microbiol. 9, 1922–1935 (2007)
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion–from mechanism to translation. Nature Med. 17, 1391–1401 (2011)
Eltzschig, H. K. et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood 108, 1602–1610 (2006)
Morote-Garcia, J. C., Rosenberger, P., Kuhlicke, J. & Eltzschig, H. K. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111, 5571–5580 (2008)
Schingnitz, U. et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 184, 5271–5279 (2010)
Eltzschig, H. K., Bonney, S. K. & Eckle, T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 19, 345–354 (2013)
Eckle, T. et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nature Med. 18, 774–782 (2012)
Eckle, T. et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 27, 3078–3089 (2013)
Morote-Garcia, J. C., Rosenberger, P., Nivillac, N. M., Coe, I. R. & Eltzschig, H. K. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology 136, 607–618 (2009)
Frick, J. S. et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J. Immunol. 182, 4957–4964 (2009)
Csoka, B. et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 185, 542–550 (2010)
Belikoff, B. G. et al. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J. Immunol. 186, 2444–2453 (2011)
Zhang, K. et al. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature http://dx.doi.org/10.1038/nature13083 (23 March, 2014)
Zhang, J. et al. Agonist-bound structure of the human P2Y12 receptor. Nature (in the press).
Acknowledgements
We acknowledge S. A. Eltzschig for help with artwork during manuscript preparation. The present research is supported by Deutsche Forschungsgemeinschaft grant ID7/3-1 ID7/4-1 and a grant by the Boehringer-Ingelheim Foundation to I.D., as well as National Institutes of Health grants R01-DK097075, R01-HL0921, R01-DK083385, R01-HL098294, POIHL114457-01 and a grant by the Crohn’s and Colitis Foundation of America to H.K.E.
Author information
Authors and Affiliations
Contributions
M.I., D.F. and H.K.E. all contributed to the writing of this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary References. (PDF 519 kb)
Rights and permissions
About this article
Cite this article
Idzko, M., Ferrari, D. & Eltzschig, H. Nucleotide signalling during inflammation. Nature 509, 310–317 (2014). https://doi.org/10.1038/nature13085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13085
This article is cited by
-
Differential contribution of THIK-1 K+ channels and P2X7 receptors to ATP-mediated neuroinflammation by human microglia
Journal of Neuroinflammation (2024)
-
The microRNA-211-5p/P2RX7/ERK/GPX4 axis regulates epilepsy-associated neuronal ferroptosis and oxidative stress
Journal of Neuroinflammation (2024)
-
Rationale for sequential extracorporeal therapy (SET) in sepsis
Critical Care (2023)
-
P2X1 enhances leukemogenesis through PBX3-BCAT1 pathways
Leukemia (2023)
-
Roles of extracellular adenosine triphosphate on the functions of periodontal ligament cells
BDJ Open (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.